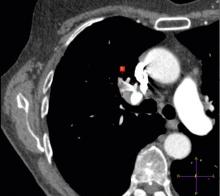
TeraRecon unveiled the latest release of the company’s flagship iNtuition enterprise viewing solutions in combination with the second-generation of its iNteract+ interoperability platform at the annual European Congress of Radiology (ECR) meeting, March 4-8 in Vienna.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now