November 9, 2017 — Abbott received European CE mark for Xience Sierra, the newest generation of the company's Xience everolimus-eluting coronary stent system. CE mark allows sale of the device in the European Union and other countries that recognize CE mark. Advances in this generation of Xience include new features that make it easier for cardiologists to successfully complete complex procedures that now account for up to 70 percent of cases.[2]
"Doctors tell us they need better tools to treat increasingly challenging cases, which involve multiple, or totally blocked arteries and complications such as diabetes," said Chuck Brynelsen, senior vice president of Abbott's vascular business. "We designed Xience Sierra with the goal of helping more people with coronary artery disease regain their health and return to their daily lives as quickly as possible."
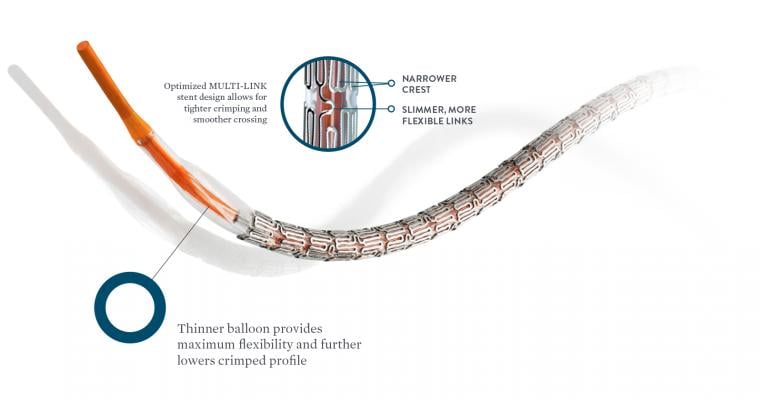
Xience Sierra makes it easier for cardiologists to access and unblock difficult-to-reach lesions. New features include a thinner profile, increased flexibility, longer lengths, and small-diameters.
"Xience Sierra can help cardiologists be even more precise when implanting the stent, which is important for efficacy and safety," said Charles Simonton, M.D., chief medical officer of Abbott's vascular business. "Its design, range of sizes, and increased flexibility mean doctors don't have to use as much force when they implant a Xience Sierra stent compared to other stents."[3]
More than 8 million people worldwide have received a Xience stent since its initial regulatory approval.[4] It is the most commonly used stent in Europe and has been studied in over 100 clinical trials and in 10 years of global real-world experience.
Abbott has also submitted an application to the U.S. Food and Drug Administration for Xience Sierra approval in the United States.
Read the article “Abbott Will End Sales of Absorb Bioresorbable Stent.” Abbott said it abandoned sales of its bioresorable stent to concentrate efforts on the new Xience Sierra.
For more information: www.XienceStent.com
References:
1. EUCOMED and Decision Resources Group, September 2017
2. Decision Resources Group, July 2017. Data on file at Abbott.
3. Tests performed by and data on file at Abbott.
4. Data on file at Abbott.
6. Palmerini, et al. XIENCE showed significant benefit compared to several DES and composite BMS in multiple large scale meta-analyses and other RCTs. The Lancet. 379:9824, 14-20 April 2012, pp. 1393-1402; Bangalore S, et al. Circ Cardiovasc Interv, Aug 6, 2013. doi: 10.1161/ circinterventions.113.000415.; Valgimigli, et al. Effects of Cobalt-chromium Everolimus eluting or bare metal stent on fatal and non-fatal cardiovascular events. A patient-level meta analysis. EuroPCR 2014; Serruys, PW et al. RESOLUTE All Comers Trial, NEJM 2010. Published online June 16, 2010; Fajadet, J., et al. PLATINUM PLUS 30-day Poster, TCT 2012.
7. Xience showed significant benefit compared to several DES and composite BMS in multiple large scale meta-analyses and other RCTs. Palmerini, et al. The Lancet. 379:9824, 14-20 April 2012, pp. 1393-1402; Bangalore S, et al. Circ Cardiovasc Interv, Aug 6, 2013. doi: 10.1161/ circinterventions.113.000415.; Valgimigli, Effects of Cobalt-chromium Everolimus eluting or bare metal stent on fatal and non-fatal cardiovascular events. A patient-level meta analysis. EuroPCR 2014; Serruys, PW et al. RESOLUTE All Comers Trial, NEJM 2010. Published online June 16, 2010; Fajadet, J., et al. PLATINUM PLUS 30-day Poster, TCT 2012.


 November 14, 2025
November 14, 2025 









