January 11, 2017 — Avinger Inc. recently announced the U.S. launch of an enhanced version of the company’s Lightbox imaging console. The Lightbox provides a dual display of images to physicians using Avinger’s Lumivascular system, the first-ever image-guided atherectomy and chronic total occlusion (CTO) recanalization devices for the treatment of peripheral artery disease (PAD), providing physicians with a view inside diseased arteries. The upgraded Lightbox L250 console is designed to be more intuitive, provide sharper imaging and reduce start-up times before procedures begin.
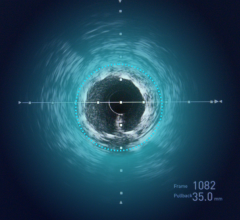
Atherectomy is a minimally invasive treatment for PAD in which a catheter-based device is used to remove plaque from a blood vessel. Lumivascular technology allows physicians to see from inside the artery during a directional atherectomy procedure by using an imaging modality called optical coherence tomography (OCT) that is displayed on the Lightbox console. In the past, physicians have had to rely solely on X-ray as well as tactile feedback to guide their tools while treating complicated arterial disease. With the Lumivascular approach, physicians can more accurately navigate their devices and treat PAD lesions, thanks to the real-time OCT images generated from inside the artery, without exposing healthcare workers and patients to the negative effects of ionizing radiation.
Specific enhancements to the Lightbox L250 include:
- Digital Video Output: The addition of an HD video output via HDMI allows for deeper integration of Avinger’s OCT imaging technology into the cath lab environment, allowing multiple images to be displayed simultaneously on existing imaging banks and providing a more seamless workflow of treatment for PAD patients;
- Touch Screen Interface: A new 10-point touchscreen display replaces legacy mouse and keyboard input for increased ease of use and efficiency during procedures; and
- Simplified Software Workflow: With an emphasis on touch input, the software user interface and workflow have been streamlined with a focus on efficiency. For example, users can now go from powering on the Lightbox to imaging ready in two taps of the touch screen.
All upgrades and updates available in the Lightbox L250 will be fully transferable to Avinger’s previous Lightbox models.
For more information: www.avinger.com

 December 15, 2025
December 15, 2025