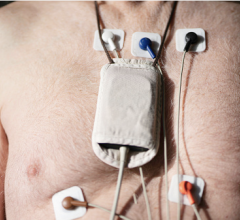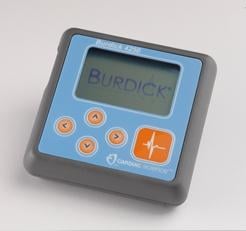
October 15, 2012 — Cardiac Science has unveiled its Burdick 4250 Holter recorder — a wearable heart monitor half the size and weight of its Holter monitoring system predecessor.
The smaller recorder offers the same functionality as the popular Burdick Vision 5L Holter recorder, including high-quality five- or seven-lead three-channel recordings, and features an ECG signal preview screen. Using the new Vision 3.5 software platform released with the recorder, clinicians will be able to view test data on widescreen monitors.
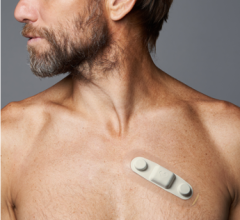
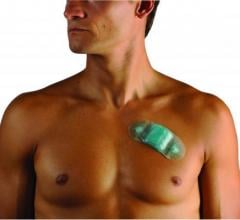
"Patients will appreciate the Burdick 4250's compact design that allows them to wear the recorder comfortably and unobtrusively under clothing, making it easier for them to complete cardiac studies," said Ben Swahn, general manager of diagnostic cardiology at Cardiac Science. "Clinicians will appreciate the increase in quality of clinical data and the decrease in re-testing."
The Burdick 4250 offers convenient options for downloading data from the recorder to computers for clinical analysis, including a secure data (SD) card and USB connection. Rapid data download allows busy practices to quickly reassign a recorder to the next patient. The new recorder pairs with existing Burdick Vision Holter and Vision Premier Holter analysis software, as well as with HeartCentrix, the Cardiac Science software solution used to interface with a variety of electronic health records (EHR) systems.
For information: www.cardiacscience.com


 March 31, 2025
March 31, 2025