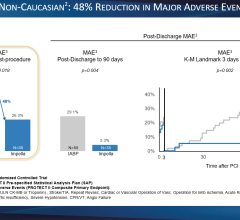
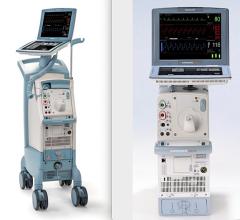
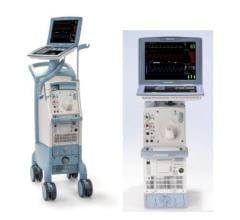
Datascope’s CS100 Intra-Aortic Balloon Pump with IntelliSync offers one-button start up and it automatically adapts to changing conditions.
The device automatically evaluates and selects optimal trigger source and lead, sets inflation at the dicrotic notch and deflates the balloon at the start of systole. It responds to changes in signal quality by selecting a new trigger source.
Datascope said the system offers consistent support for patients with PVCs and arrhythmias, has fast pneumatics, eliminates alarms due to ESU noise, and is easy to use to educed need for user intervention.
The device is marketed for use in the cath lab, CCU the OR.
For more information: www.datascope.com

 August 14, 2023
August 14, 2023