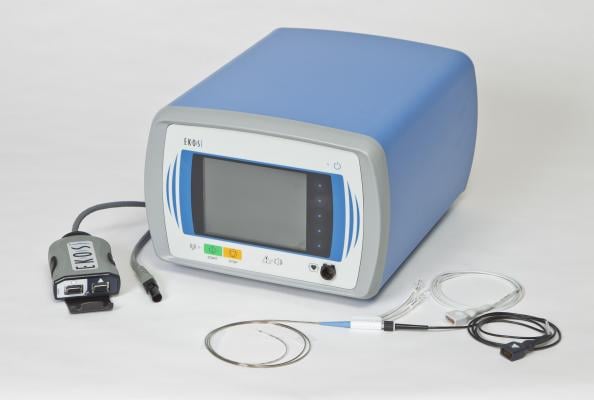

May 27, 2014 — Ekos Corp. announced the U.S. Food and Drug Administration (FDA) has cleared the EkoSonic endovascular system for the ultrasound-facilitated, controlled and selective infusion of physician-specified fluids, including thrombolytics, into the vasculature for the treatment of pulmonary embolism (PE).
The Ekos ultrasonic devices are designed to gently accelerate the penetration of thrombolytic agents into thrombus, providing high levels of lysis. Ekos is the only minimally invasive endovascular therapy on the market that has been FDA-cleared for the treatment of PE.
PE occurs in approximately 600,000 patients in the United States, causing or contributing to 200,000 deaths each year. PE is also said to cause or contribute to 15 percent of all hospital deaths. Samuel Z. Goldhaber, M.D., professor of medicine, Harvard Medical School, director, Thrombosis Research Group, Brigham and Woman’s Hospital, said, “The Ekos clinical data established that patients stricken with a life-threatening pulmonary embolism can be successfully and safely treated with the EkoSonic system. This is the first FDA-cleared treatment option for PE since the approval of the drug tPA in 1990.”
“Interventional radiologists, cardiologists, cardiothoracic and vascular surgeons at leading institutions around the world use our system to provide faster, safer and more complete dissolution of thrombus,” said Matt Stupfel, general manager of Ekos. “We are proud to have completed the world’s only randomized controlled trial (ULTIMA) and the largest prospective single-arm trial (SEATTLE II) on the safety and effectiveness of Ekos therapy in the treatment of PE. The positive outcomes of those trials, combined with our expanded indication, will allow a better standard of care for thousands of patients who suffer from PE.”
In January 2014, the outcomes of ULTIMA were published in American Heart Association’s journal Circulation. The trial demonstrated that for PE patients at intermediate risk of adverse events, Ekos treatment was clinically superior to anticoagulation with heparin alone in reversing right ventricular dilation at 24 hours, without an increase in bleeding complications.
Following ULTIMA, the results of SEATTLE II, the prospective single-arm multicenter trial of 150 patients, were released at this year’s American College of Cardiology (ACC) annual meeting. The SEATTLE II trial demonstrated that ultrasound-facilitated catheter-directed low-dose fibrinolysis for acute PE minimizes the risk of intracranial hemorrhage, improves RV function and decreases pulmonary hypertension.
For more information: www.ekoscorp.com

 October 27, 2025
October 27, 2025