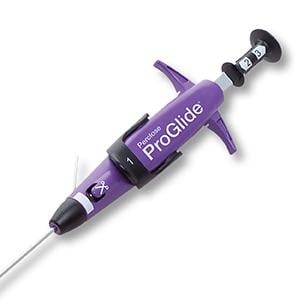
April 17, 2018 — The U.S. Food and Drug Administration (FDA) announced market approval for the Abbott Perclose ProGlide Suture-Mediated Closure System, designed to deliver a single suture to close the access sites in large vessels in the leg following catheterization procedures.
The ProGlide is used after the insertion of large-bore catheters and is able to close access sites ranging from about 0.07 inches to 0.32 inches in diameter. The system is composed of a plunger, handle, guidewire and sheath. The surgeon places the ProGlide over a guidewire and inserts the end of the device into the blood vessel. The surgeon presses the plunger and lever on the handle of the device, which maneuver the suture to create a stitch across the access site.
In some cases (3 out of 10), in addition to the ProGlide stitch, pressure may need to be applied to the access site to fully stop blood flow. When catheters larger than about 0.1 inches in diameter are used during the catheterization procedure, two ProGlide devices are necessary to close the access site.
For more information: www.abbottvascular.com


 November 14, 2025
November 14, 2025 









