
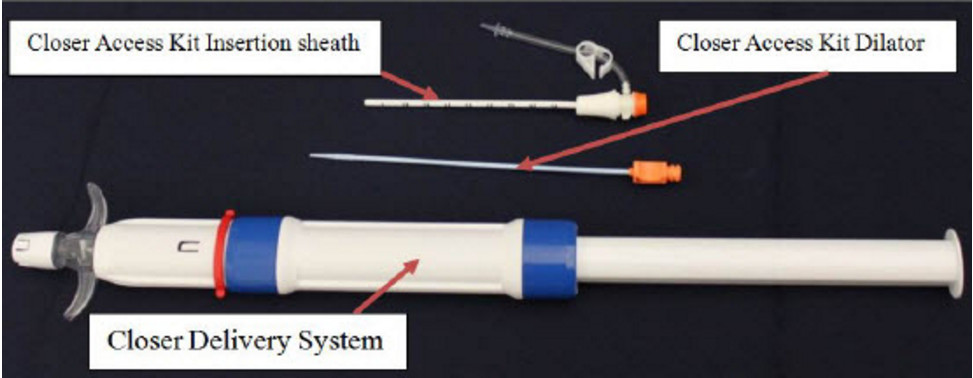
August 3, 2016 — The U.S. Food and Drug Administration (FDA) has granted market clearance to Rex Medical’s bioresorbable Closer Vascular Sealing System (VSS) to achieve rapid hemostasis of femoral artery catheterizations.
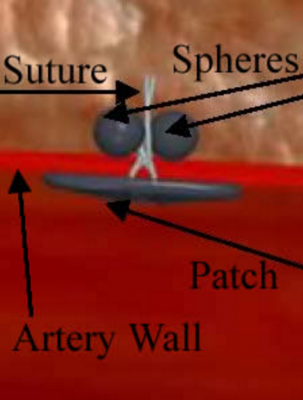
The vascular closure device consists of an insertion sheath, dilator and an implant contained in a delivery system. It uses a patch placed against the artery wall and is secured on the outside of the vessel by an an attached suture connected to two spheres. The suture brings the patch and spheres together. The patch and spheres are made of polylactide-co-glycolide acid co-polymer. The suture is made of Polydiaxanone. The components of the device are designed to dissolve inside the body after they serve their purpose.
Doctors put the sheath into the artery through the hole in the artery wall. They then use the delivery system to push the patch out of the end of the sheath into the artery. As the doctor pulls the system out of the artery, the patch sits against the arterial wall. Once this happens, the spheres are uncovered in the space outside the artery. Finally, the patch and spheres are “cinched” together such that the device sandwiches the arterial wall. This stops the blood flow. Later, the body absorbs the patch, sutures and spheres after the wound heals.
The system helps enable faster ambulation of the patient, reducing recovery and nursing time.
For more information: rexmedical.com


 November 14, 2025
November 14, 2025 









