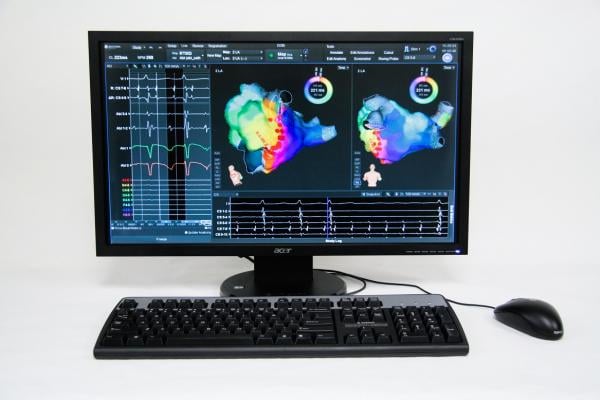
August 1, 2013 — Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the Rhythmia mapping system, a next-generation 3-D mapping and navigation solution for use in cardiac catheter ablations and other electrophysiology (EP) procedures to diagnose or treat a variety of conditions in which the heart beats abnormally.
Cardiac mapping has become a standard tool for the diagnosis and treatment of arrhythmias. Current mapping systems require a manual, labor-intensive process to create maps, making tradeoffs between accuracy and speed. Current systems also offer limited indication of therapy success. The Rhythmia mapping system is designed to intelligently automate map creation, increasing the speed and improving the density of mapping compared to existing systems. The system also features vMap, a validation map, which is designed to enable electrophysiologists to rapidly confirm the endpoints of the ablation treatment for the first time.
"I believe the Rhythmia mapping system will become the new gold standard for mapping and navigation," said Warren Jackman, M.D, professor of medicine, University of Oklahoma Health Sciences Center. "Rhythmia delivers maps of exceptional clarity because it captures thousands versus hundreds of data points. The magic of Rhythmia is continuous mapping. The intelligence built into the system virtually eliminates the need for manual annotation, which is expected to facilitate the diagnosis, treatment and final verification of arrhythmias."
Boston Scientific is offering the Rhythmia mapping system with the company's 64-electrode IntellaMap Orion high-resolution mapping catheter, which has also received FDA 510(k) clearance.
"We are committed to developing technologies that surround electrophysiologists with innovative solutions designed to meaningfully improve patient outcomes and increase operational efficiencies," said Pete Sommerness, general manager, EP, Boston Scientific. "Extending the reach of the Rhythmia mapping system to electrophysiologists in the [United States] marks another significant milestone in our journey to redefine cardiac arrhythmia ablation management. We believe this clearance will help Boston Scientific achieve scale and scope to better serve the rapidly growing global EP market."
The Rhythmia mapping system received CE mark approval in May 2013.
For more information: www.bostonscientific.com


 January 29, 2026
January 29, 2026 









