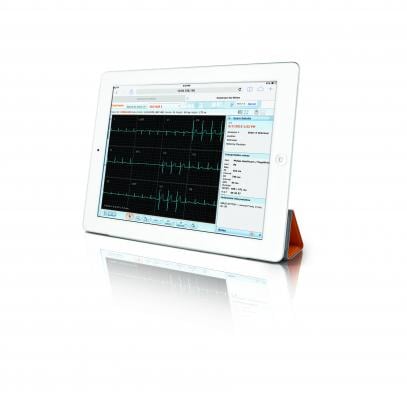
November 20, 2015 — Carestream has obtained U.S. Food and Drug Administration (FDA) clearance for diagnostic reading of electrocardiogram (ECG) waveforms on desktop displays and mobile tablets using its Vue Motion universal viewer. Emergency room physicians, cardiologists and other clinicians can quickly and easily view ECG exams to treat patients with ST segment elevation myocardial infarction (STEMI) as well as other heart conditions and illnesses. This capability is also available as an optional module in Carestream’s Vue PACS Client.
“Patients suffering from chest pain can face death or disability if their condition is not assessed and treated immediately,” said Ron Muscosky, Carestream’s Worldwide Product Line Manager for Healthcare Information Solutions. “Equipping ER physicians, cardiologists or other specialists with the ability to view ECG waveforms from virtually anywhere in seconds delivers valuable diagnostic information that can enhance patient care. This is especially important for a STEMI case, which is a severe and deadly type of heart attack that is identified by a specific ECG pattern.”
The new capabilities allow physicians to simultaneously view current and prior ECGs using tools that include pan, zoom, line measurement, caliper, and gain and speed adjustments.
Carestream’s zero footprint Vue Motion viewer is also FDA-approved for diagnostic viewing of imaging exams on approved mobile devices. It equips clinicians with “one-click” access to reports that contain embedded hyperlinks and bookmarks for quick and easy viewing of anatomical regions of interest. In addition, Vue Motion enables data sharing so remote physicians and specialists can conveniently access 3-D/MPR (multi-planar reformatting) images and non-DICOM data such as videos.
For more information: www.carestream.com


 November 06, 2025
November 06, 2025