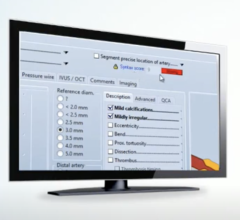
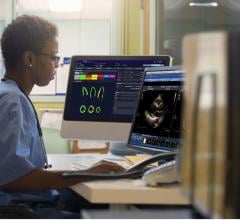
August 27, 2014 — Fysicon received approval from the U.S. Food and Drug Administration (FDA) to market Fysicon DataLinQ. The Fysicon DataLinQ products enable complete automation. The DataLinQ Pacemaker Programmer Acquisition Device (2PAD) collects complex specialist data from every pacemaker/ICD programmer or remote care, and integrates this data into an Electronic Medical Record (EMR) or PACS-2. DataLinQ Cardiac Rhythm Management (CRM) takes care of the storage of implants and follow-ups for both Pacemakers and ICDs.
Complex specialist data processing systems are notoriously cumbersome and difficult to link directly into an electronic medical record (EMR) or PACS-2. Compatibility problems between systems often make integration impossible. As a result, exchanging information often takes place by unsecured e-mail, letter or fax and in rare cases it is even transcribed. The resulting productivity loss can lead to costly medical errors. DataLinQ quite simply provides the missing link for all common integration problems between front-line complex data processing systems and healthcare informatics systems.
“The approval of DataLinQ is important news for physicians who need new options for data processing, and it is a significant milestone for Fysicon,” said Bert Elberse, president and CEO of Fysicon. “This marks a major achievement towards our goal of providing a significant contribution to improving healthcare.”
For more information: www.fysicon.com


 November 06, 2025
November 06, 2025