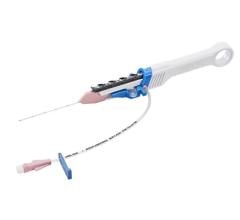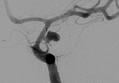
August 17, 2009 - Reverse Medical Corporation announced that it has received U.S. FDA 510k clearance for its ReCruit Microcatheter, intended to retrieve intravascular foreign objects during interventional radiology procedures, including from the neurovasculature.
Reverse Medical scientific and clinical advisor Dr. Satoshi Tateshima, M.D., D.M.Sc., UCLA Stroke Center, Ronald Reagan UCLA Medical Center, Los Angeles, CA stated, “I believe the Reverse Medical ReCruit Microcatheter is a very innovative device that will serve as a platform technology for even more advanced interventional neurovascular devices. As a neuro interventionalist, the ability for me to control the ReCruit Microcatheter’s distal tip rate of radial deployment is quite unique and represents a significant improvement over other devices I’ve used.” Dr. Tateshima added that the device’s flexibility allows for deep brain navigation through tortuous anatomy.
The approval allows the company to market the ReCruit Microcatheter in the United States for commercial distribution purposes. With the regulatory clearance for U.S. marketing, initial clinical use will be closely managed to collect data demonstrating the clinical value of the ReCruit compared to competitive products before the company markets the product on a broad scale. The company expects to commence commercialization in early 2010.”


 January 29, 2026
January 29, 2026