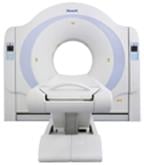
February 23, 2009 - Neuisys Imaging System Solutions announced that the FDA has granted 510(k) clearance of the NeuViz 16, a multislice computed tomography (CT) system, which was developed by Philips Neusoft Medical Systems, a joint venture between Royal Philips Electronics of the Netherlands and Neusoft Corporation of China. The NeuViz 16 is said to provide sub-millimeter resolution, volume imaging and low-dose protocols, all in an economical footprint. The system is suited for diagnostic imaging centers and community hospitals that plan to upgrade from older single or multi-slice CT systems to 16-slice CT as well as a wide array of specialist physician groups considering in-office CT including urologists, gastroenterologists, pulmonologists, surgeons and oncologists. "With the NeuViz 16, physician groups can now routinely provide their patients with convenient, highly advanced in-office CT imaging, shortening the stressful time between testing and diagnosis report to the patient," said Joseph Jenkins, M.D., Neuisys chief medical officer. "The NeuViz 16 provides many advanced clinical applications including CT angiography of peripheral and neuro vasculature, virtual colonoscopy, lung nodule protocols, pulmonary embolism protocols, CT urography and tissue perfusion studies." For more information: www.neuisys.com


 February 02, 2026
February 02, 2026 









