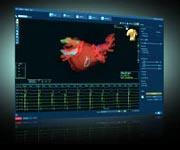
September 15, 2009 – St. Jude Medical Inc. said yesterday the U.S. Food and Drug Administration (FDA) cleared and U.S. launch of the EnSite Velocity Cardiac Mapping System, which is designed to help physicians more efficiently diagnose and guide therapy to treat abnormal heart rhythms.
Advancements in the design of the EnSite Velocity System increase procedural efficiency and speed, making it easier to use from set-up and operation, to clinical application, the company said. With new hardware and software, the system offers simple set-up and connections, an intuitive software interface and includes two key new capabilities, the OneMap tool and RealReview function. The OneMap tool enables physicians to create a detailed cardiac model and electrical map together using multiple catheters and electrodes, allowing physicians to collect and display more relevant patient information in a shorter amount of time. The RealReview function provides real-time, side-by-side views of the live procedure and previously recorded portions of the procedure. This feature gives physicians a quick and easy comparison of events and results at different times throughout the procedure, without losing the ability to visualize and navigate catheters in real-time. The EnSite Velocity System is an open platform, which means that it is compatible with essentially all diagnostic and ablation catheters, recording systems and energy sources used for ablation procedures.
“We have been using the EnSite System for nearly 10 years because of its ability to display and map electrical signals from multiple catheters and electrodes, and its convenience of being an open platform,” said Walid Saliba, M.D., of the Cleveland Clinic Foundation in Cleveland, Ohio. “The next-generation EnSite Velocity System also allows us to treat patients with any arrhythmia and navigate the heart with potential reduction in procedural time.”
St. Jude said the system helps reduce fluoroscopy exposure and is the only mapping system capable of supporting both contact and noncontact mapping.
“This system has exceptionally detailed chamber models and clear electroanatomical maps,” said Javier Sanchez, M.D., of the Texas Cardiac Arrhythmia Institute at St. David's Medical Center in Austin, Texas. “Compared to other systems, I found the user interface to be very intuitive, quick and easy to learn.”
The EnSite Velocity System is used in minimally invasive electrophysiology procedures. Catheters with electrodes are inserted into a cardiac chamber; these electrodes are then located or visualized by the system, which records electrical information from the heart and displays it in a three-dimensional anatomical model. These highly detailed anatomical models, or maps, enable physicians to diagnose and guide therapy for abnormal heart rhythms. Like previous models of the EnSite System, the EnSite Velocity System allows catheter navigation to occur with reduced fluoroscopy, thus reducing potential for risks associated with excessive exposure to X-rays.
For more information: www.sjm.com


 January 29, 2026
January 29, 2026 









