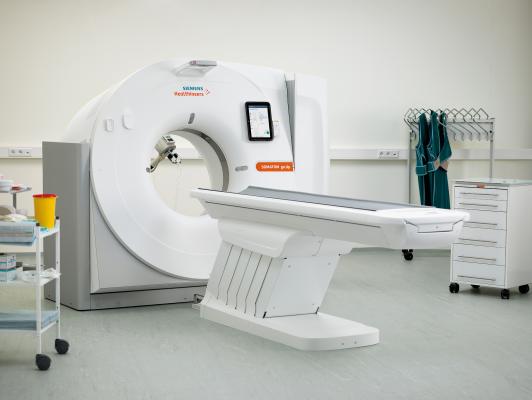
April 11, 2017 — The U.S. Food and Drug Administration (FDA) has cleared the Somatom go. computed tomography (CT) platform from Siemens Healthineers, designed for highly diverse sets of user needs.
The new platform includes the Somatom go.Now and Somatom go.Up CT scanners, which offer automated, standardized workflows that help users achieve more consistent clinical results at a lower total cost of ownership. The tablet-based workflow concept facilitates more comprehensive patient care and allows radiology providers to use a cost-efficient single-room concept for the first time, according to Siemens.
The 32-slice Somatom go.Now CT scanner is particularly suited to radiology providers who want to leverage a low-cost but clinically robust CT solution. With a wide detector that provides up to 64 slices, the Somatom go.Up offers faster scanning and tin filtration, which is particularly important for lung imaging (e.g., to screen for lung cancer). It also uses some of the lowest radiation doses achievable for a CT of this class, according to the company.
Users of the Somatom go. platform can control routine examinations using only the tablet and remote, paving the way for a new, mobile workflow. Standardized work steps allow users to run the scan with just a few inputs. Automated post-processing facilitates efficient scanner operation with zero-click reconstruction tasks that enable technologists with wide-ranging proficiency to generate consistent, high-quality studies. The standardization also provides radiologists with greater assurance regarding diagnostic image quality, helping them avoid errors and repeat scans, and thus unnecessary wait times.
Since workflow can be controlled via tablet, staff must no longer move between the scanner and control room. Technologists can remain with patients during scan preparation, which can make the experience more pleasant, particularly for children.
Since all computer hardware formerly located in the control room is now integrated into the gantry, Somatom go. scanners can be controlled on the move, enabling a flexible room concept. Rather than require two or three rooms for the scanner, the control unit, and possibly additional technology, the systems can be installed in one room with a minimum size requirement. With this setup, a shielded niche can sufficiently protect radiology staff. This room concept drastically reduces installation costs.
The platform’s holistic service approach — the Siemens Healthineers Connect Plan — focuses on remote diagnostic and service capabilities, online training and novel concepts for extended spare parts coverage. This solution optimizes financial performance and enables highly reliable operation. For example, the new Chronon X-ray tube is designed for a long lifespan to relieve customers’ financial burden. Remote service also significantly reduces downtime, and customers can install many upgrades remotely at their discretion without disrupting scanner operation.
The clinically relevant features of the Somatom go. platform are particularly important for many routine applications. A new Stellar detector with integrated electronics, coupled with spectral tin filters previously available only on the company’s most advanced single- and dual-source CT scanners, are included as standard. For vascular imaging, the Chronon tube is designed to deliver High Power 80, which uses low 80 kV tube voltages at high mA to potentially reduce radiation dose and optimize usage of iodine contrast.
For more information: www.usa.healthcare.siemens.com


 February 02, 2026
February 02, 2026 









