Morris Innovative offers the FISH (Femoral Introducer Sheath and Hemostasis) Device that combines one sheath as both an introducer and closure device.
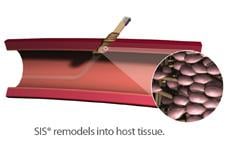
The FISH utilizes a sleeve of extracellular matrix, known as small intestinal submucosa (SIS), pre-mounted on a sheath introducer to serve as the closure mechanism. The SIS is remodeled into native arterial wall tissue in about 30 days, the company said. Instead of the formation of scar tissue, viable host tissue is formed at the arteriotomy with the use of the FISH device. The company said this allows easy reaccess during future procedures.
Because FISH uses a single introducer and hemostasis sheath, the time for deployment and the time required in the catheterization lab at the conclusion of the diagnostic or interventional procedure should be dramatically reduced, the manufacturer said.
Follow up interviews with patients participating in the clinical trial of the device indicated minimal patient discomfort. FISH works from inside the vessel, enhancing clotting mechanisms, while avoiding impingement and injury to the nerve tissue that resides on the external surface of and adjacent to the vessel. The clinical trials also showed significant reductions in time to hemostasis and time to ambulation for the patients.
The complication rate of the device after 200 patients receiving the device is 0.5 percent. The company said this rate is among the lowest for this type of trial and can be attributed to the reversibility of the device, as well as the infection resistant properties of the SIS. In the event of a complication, FISH biomaterial can easily be removed by using the safety sutures, and administering manual compression to close the arterial opening.
The company launches the device in October 2008.


 October 07, 2025
October 07, 2025 









