October 20, 2011 – GE Healthcare’s CT and Advantage Workstation team at RSNA 2011 will showcase innovative new products and technologies that may enable physicians to diagnose more confidently while improving image clarity, reducing patient dose and enhancing workflow.
Specifically:
• The Dexus workflow environment, offering improved efficiency through unique PACS integration
• The recently 510(k)-cleared Optima CT660 64-slice system, providing a scalable, low dose platform that enables fast, high performance imaging in a variety of clinical settings. Optima CT660 is also the first CT system that is both ecomagination and healthymagination-certified, two of the GE’s global initiatives aimed at transforming healthcare delivery through innovation, partnerships and sustainability
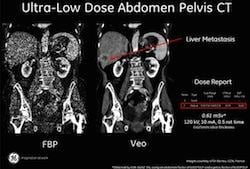
Now available in the United States, GE’s revolutionary reconstruction technology, called Veo, is the industry’s first model based iterative reconstruction (MBIR) technology. Veo capabilities change the rules of CT imaging by offering superb image clarity at dramatically low dose level, and represent GE’s promise to help eliminate the traditional trade-off between image clarity for the physician and low-dose for the patient. In fact, existing Veo users have demonstrated sub- millisievert CT imaging with profound image clarity
New dose alert and monitoring tools, such as Dose Check, will also be on display, highlighting GE’s continued commitment to low dose imaging
For more information: www.gehealthcare.com


 February 02, 2026
February 02, 2026 









