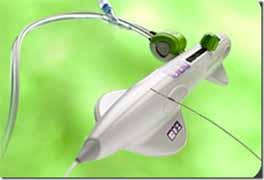
August 4, 2010 — A second-generation of the Diamondback Predator 360 peripheral arterial disease (PAD) system features improvements in the crowns and shaft for enhanced clinical performance and shorter procedure times. The U.S. Food and Drug Administration (FDA) recently granted Cardiovascular Systems Inc. (CSI) special 510(k) marketing clearance for the device.
The Diamondback Predator 360 is a minimally invasive catheter system offering physicians a first-line therapy to quickly change lesion compliance and facilitate low-pressure balloon inflation. The device can also create a smooth vessel lumen without placing a stent. The system includes a diamond-coated crown and unique orbital mechanism of action to remove hardened plaque to restore blood flow in arteries throughout the leg. The core technology of these systems is designed to avoid damage to blood vessels to possibly delay restenosis and foster better long-term patient outcomes.
“Cardiovascular Systems’ PAD solutions are highly effective in removing plaque from arteries, and the Diamondback Predator 360 enhances the treatment process,” said Prakash Makam, M.D., medical director of clinical research at Community Hospital in Munster, Ind. “The ability to use lower speeds reduces the risk of complications during the procedure while removing a high percentage of plaque to achieve excellent acute outcomes in less time.”
CSI has also initiated the CONFIRM PREDATOR registry, a prospective, multicenter study that will follow 500 PAD patients treated with the Diamondback Predator 360. The new registry will collect information on Diamondback Predator 360 performance, including: overall plaque reduction, key acute safety measures and techniques for optimal outcomes.
For more information: www.csi360.com


 November 08, 2024
November 08, 2024 









