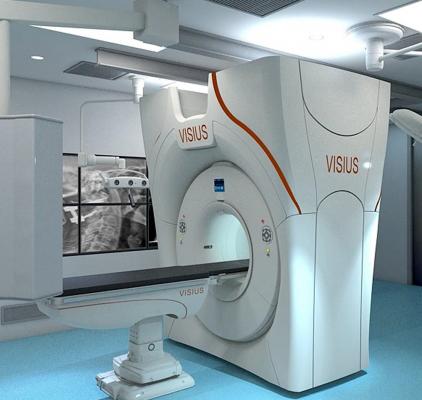
October 10, 2013 — IMRIS Inc. and Siemens Healthcare announced an agreement that positions one of Siemens’s computed tomography (CT) products as the core of the IMRIS Visius iCT solution.
“Siemens and IMRIS have successfully collaborated on solutions for intraoperative magnetic resonance imaging (MRI) and angiography, so adding CT is a natural extension of this relationship,” said Murat Gungor, vice president, CT and radiation oncology business unit, Siemens Healthcare North America. “We are looking forward to bringing the established benefits of low-dose CT imaging to the intraoperative environment.”
Developed for the neurosurgery and spine surgery markets, the Visius iCT contains a 64- slice Siemens Somatom Definition AS scanner at its core.
The Visius iCT moves into and out of the operating room (OR) in less than 30 seconds using ceiling-mounted rails and features software applications that maximize image quality while minimizing radiation exposure in real time and post-processing software to enhance surgical diagnosis and intervention.
Visius iCT enables multiple operating room configurations to meet clinical requirements and increase system utilization. Layouts include one or two OR configurations with a center room isolating the CT from the surgical theater while not in use.
IMRIS received U.S. Food and Drug Administration (FDA) clearance for Visius iCT in July. The company currently has systems at three eastern U.S. neurosciences centers.
For more information: www.imris.com


 February 02, 2026
February 02, 2026 









