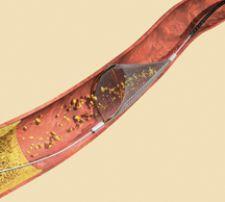
The SpideRX Embolic Protection Device features a preloaded nitinol filter with a dual-ended catheter for delivery and recovery. Offering a fully rapid exchange system, the device is easy to use in coronary, carotid and peripheral interventions.
The device enables use of a preferred 0.014 to 0.018-inch guide wire to reach and cross the lesion, and a single dual-ended low-profile catheter — one end for delivery and the opposite end for recovery. The preloaded filter/capture wire is ready to flush and deploy with no stylet or introducer.
With a 6F guide catheter compatible for all SpideRX filter sizes, the system boasts a 3.2F crossing profile, enabling minimal interaction with the lesion during placement. Multiple filter sizes (3.0, 4.0, 5.0, 6.0, 7.0mm) allow optimal matching of the filter to the vessel size.


 April 25, 2023
April 25, 2023 









