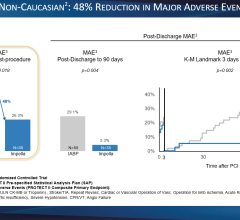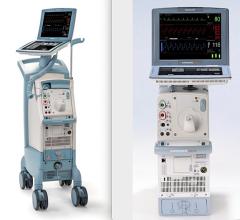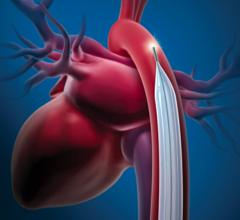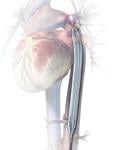
October 22, 2009 – MAQUET Cardiovascular LLC said today it received European CE mark for the company's MEGA 8 Fr. 50 cc intra-aortic balloon (IAB) catheter. The company said it is the world's first 50 cc IAB with a true 8 Fr. shaft, and the MEGA has a smaller insertion point than traditional 50 cc IABs and offers additional blood volume displacement for intra-aortic balloon counterpulsation. MEGA, compared to 40 cc IABs, delivers 25 percent more blood volume displacement and provides improved unloading and augmentation MEGA balloon's impact in patient care was recently noted in the clinical setting. "I recently admitted a critically ill approximately 180 kg patient and inserted the MEGA 50 cc balloon," said George W. Christy, MD, FACC, interventional cardiologist with Advocate Christ Medical Center's Transplant and Assist Device team in Oak Lawn, Ill. "The increased augmentation we observed from MEGA, allowed us to stabilize the patient." Dr. Christy believes that "the MEGA gave us the needed window of time to consider further alternatives to manage this patient." MAQUET Cardiovascular recently acquired Datascope, a distinguished provider of gold-standard IABs and cardiac assist products. By uniting forces, MAQUET is even better positioned to advance patient care technology through innovations like the MEGA. Intra-aortic balloon counterpulsation is an adjunctive therapy that is often used in patients with left ventricular failure and other cardiac conditions. When the IAB inserted into the patient's aorta counterpulsates with the heart, it augments coronary blood flow to increase myocardial oxygen supply and decrease myocardial oxygen demand. "Since the MEGA is indicated for patients 162 cm (5-foot, 4 inches) and taller, I would generally use it as my standard balloon moving forward," adds Dr. Christy. "It's straightforward, easy to insert, and compatible with all the standard consoles." For more information: www.maquet.com

 August 14, 2023
August 14, 2023