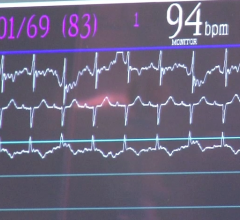
January 25, 2012 — Philips Healthcare announced the release of the Xper Flex Cardio Physiomonitoring system, a hemodynamic cardiac monitoring solution used to facilitate invasive investigation of heart and vascular disease. Equipped with advanced clinical decision support tools to assist clinicians in evaluating a patient's overall cardio performance, and general cardiovascular status, Xper Flex Cardio may also be used to display and analyze invasive blood pressure, surface electrocardiogram (ECG), respiration and other vital signs. The system is specifically designed for environments such as cath labs, EP labs and interventional radiology and cardiovascular Hybrid operating room (OR) environments.
The Xper Flex Cardio Physiomonitoring system presents several distinct advantages over previous systems, including traditional 12-lead interpretation of the resting ECG in addition to 16-lead analysis, enabling clinicians the freedom to choose which approach is most appropriate for each situation. The additional right chest and posterior leads used in 16-lead ECGs can provide information that may not be apparent on traditional 12-lead ECGs.
“Implementing Xper Flex Cardio has helped us enhance both the functionality and accuracy of our cath lab,” said Karen Keane, director, cardiac cath labs, Martin Memorial Medical Center. “Its ability to analyze up to 16-leads of simultaneously-acquired ECG waveforms enables us to refine patient care, and its smaller size makes it the perfect system for our high-use, multi-function lab.”
Xper Flex Cardio also comes equipped with integrated fractional flow reserve (FFR) functionality—which provides data that could aid in treatment decisions, and a DXL ECG Algorithm analysis capability that uses multiple steps to produce precise and consistent ECG measurements that are used to generate interpretive statements. Additionally, the system has Culprit Artery Detection—a technology that provides suggestions on the probable site of an occlusion prior to cath procedure, saving valuable time and assisting with procedure planning—and patented ST Maps that provide a graphical indication of ST elevation.
The company also stated that it entered into an agreement with Volcano Corp, to ensure seamless experience for mutual customers of the Volcano SmartMap system. Additionally, Philips has secured strong ties with FFR market leader St. Jude Medical.
For more information: www.philips.com

 January 13, 2026
January 13, 2026