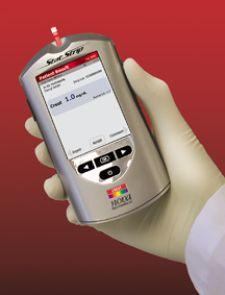
A new, hand-held creatinine monitoring system, Nova StatSensor Creatinine, was released in January by Nova Biomedical and enables simple, rapid and accurate assessment of renal function by finger prick capillary blood sampling at the point of care.
Incorporating new Multi-Well test strip technology adapted from Nova�s hospital glucose monitoring system, Nova StatSensor Creatinine allows creatinine to be measured with a simple 30-second test.
The maker said the device measures creatinine and calculates estimated glomerular filtration rate (eGFR) by MDRD or Cockroft-Gault equations. Creatinine with eGFR is a more accurate and sensitive measurement of kidney function than creatinine alone, the company said.
StatSensor Creatinine uses 1.2-microliter blood samples and delivers 30-second test results.
February 2008


 October 21, 2025
October 21, 2025 









