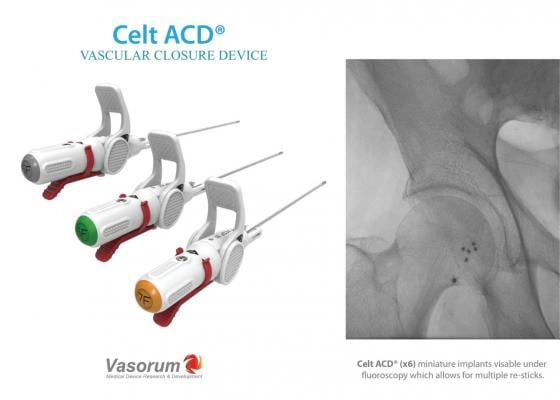
December 14, 2017 — Vasorum Ltd, the developer and manufacturer of the novel Celt ACD vascular closure device, has added a 7F-sized Celt ACD device to its range following U.S. Food and Drug Administration (FDA) approval of its premarket approval (PMA) supplement. Celt ACD is indicated for arterial puncture closure in both diagnostic and interventional cardiology and radiology patients. Vasorum said the device offers excellent time to hemostasis in a wide variety of clinical situations. The second-generation Celt ACD devices now available in both the United States and Europe have a new improved delivery system which has been designed to enhance the user experience during deployment.
The first commercial cases in the U.S. have been carried out by Shing-Chiu Wong, M.D., professor of medicine at Weill Cornell Medicine in New York. Based on his use, Wong commented that the "Celt ACD range of closure devices are addressing the clear need for quicker and more efficient methods of increasing patient throughput in healthcare facilities, and they provide doctors and patients with a solution which can efficiently manage arterial closure following vascular procedures."
Clinical cases have also been carried out by Richard Kovach, M.D., division director, interventional cardiology and medical director, cardiac catheterization laboratory at Deborah Heart and Lung, Browns Mills, N.J. Kovach commented that he "was impressed with the performance of Celt ACD in patients with severe arterial disease undergoing complex interventional procedures." Additionally, he believes that "Celt ACD has the potential to become the workhorse closure device in cath labs."
There are currently over 8 million catheter procedures performed annually, which support an estimated $1 billion femoral artery closure device market. The number of procedures is expected to exceed 10 million by 2020. In addition to interventional cardiology procedures, the market growth is being driven by an increasing number of peripheral vascular, neuro-vascular and other catheter procedures which demand more patient-friendly devices and more efficient patient discharge from hospitals.
For more information: www.vasorum.ie


 November 14, 2025
November 14, 2025 









