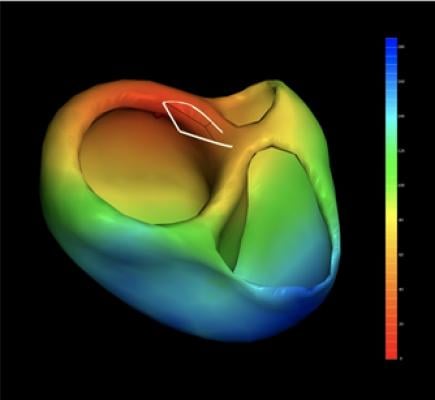
July 1, 2019 — Catheter Precision Inc. announced that the U.S. Food and Drug Administration (FDA) has cleared its new VIVO (View into Ventricular Onset) system for market release in the United States. VIVO is a pre-procedure planning tool that offers 3-D cardiac mapping to aid in localizing the sites of origin of idiopathic ventricular arrhythmias in patients with structurally normal hearts prior to electrophysiology studies. The novel VIVO system uses magnetic resonance imaging (MRI) or a computed tomography (CT) scan along with a standard 12-lead ΕCG to computer-generate color-coded 3-D mapping images of the heart to indicate the area of earliest activation.
The VIVO system consists of a handheld 3-D camera, and an off-the-shelf laptop computer with proprietary software. Using a 3-D photograph of the patient's chest, an MRI or CT (DICOM image), and a standard 12-lead ECG, the system generates a 3-D rendering of the patient's heart with superimposed color coding to indicate the area of earliest activation. VIVO uses lead positions, the chest photograph and DICOM data to determine the precise spatial relationships involved and, in that way, provide latest-generation cardiac mapping that can be used by a physician prior to conducting an electrophysiology study and subsequent ablation. The VIVO system has been shown to be 100 percent accurate (95 percent CI: 93.6 percent, 100 percent) in a prospective multi-center clinical trial.
"VIVO is an important advance for patients who may require a cardiac ablation to better understand their irregular heart rhythms," said Hari Tandri, M.D., from Johns Hopkins University Hospital. "It is easy to use, fits into our standard workflow and is not invasive. Knowing the arrhythmia foci in advance should reduce mapping time, potentially saving procedure time and cost. My experience with the VIVO to date has shown it to be an accurate, easy-to-use system that has the potential to significantly improve 3-D cardiac mapping technology for ventricular arrhythmia mapping."
For more information: www.catheterprecision.com


 February 06, 2026
February 06, 2026 









