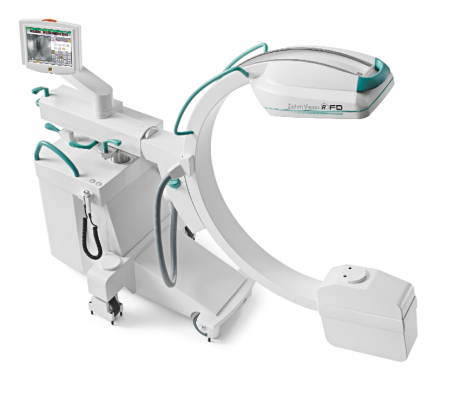
The Ziehm Vision RFD Hybrid Edition C-arm is tailored to hybrid OR requirements. It is fully motorized in four axes and features an intuitive joystick operation, distance control and maximum image quality with minimal dose levels. It also features a 20 by 20 cm flat-panel detector.
The Mobile C-arms offer a flexible, reliable and cost-effective solution for clinics with small ORs or budgetary constraints.
For more information: www.ziehm.com, www.rsna.org