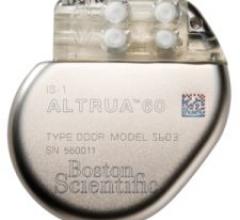
Boston Scientific's ALTRUA family of pacemakers is the first Boston Scientific-branded pacemaker…
The FDA cleared GE Healthcare’s new LightSpeed CT750 HD, said to be the world’s first high…
Northeast Monitoring received clearance from the FDA to sell its DR200 Series devices with its…
GE Healthcare launched its new mobile Vivid S5 cardiovascular ultrasound system, created for…
Textronics Inc. received FDA clearance to market its textile-based ECG Electrode for use in…
The FDA cleared for marketing a new automated version of diaDexus’ proprietary PLAC Test, an…
May 9, 2008 – Philips launched the new Xcelera R2.2, the latest version of its Xcelera…
At the 2008 American Association of Critical-Care Nurses National Teaching Institute, Holtech…
Philips offered new disposable adult ECG leadwire electrodes to help with infection control May…
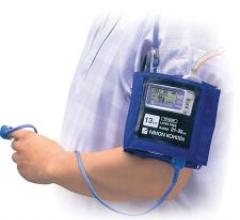
A safety engineered short-term peripheral intravenous (IV) catheter, the VantageCath, is…
CorMatrix ECM by CorMatrix Cardiovascular is for Cardiac Tissue Repair, which utilizes the…
Vascular Insights' recently 510(k) cleared ClariVein infusion catheter is designed for infusion…
May 6, 2008 - Cordis Corp. U.S.
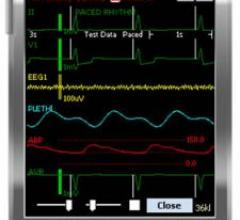
May 6, 2008 - Philips' IntelliVue mobile viewer application gives physicians access to patient…
After GE Healthcare implemented enhancements to the OEC 9900 Elite C-arm, a fluoroscopy device…
Emageon's Enterprise Content Manager (ECM) is a single, enterprise-centric application that is…
Medtronic's Defender embolic protection filter, available in Europe, is a device designed to get…
InfraReDx Inc. developed the LipiScan Coronary Imaging System that is intended to characterize…
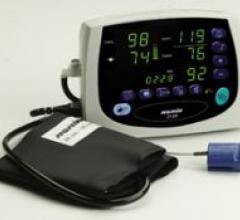
The multi-function Avant 2120 tabletop pulse oximeter combines Nonin Medical’s PureSAT pulse…

 May 12, 2008
May 12, 2008