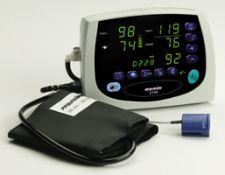
The multi-function Avant 2120 tabletop pulse oximeter combines Nonin Medical’s PureSAT pulse oximetry technology with fast, motion tolerant oscillometric NIBP.
The company said it can be used for continuous or spot-check monitoring. The Avant 2120 simultaneously measures, displays and records SPO2, pulse rate and blood pressure for patients pediatric to adult. Its compact, lightweight design makes it a highly mobile and convenient solution for hospital/sub-acute environments.
The Avant 2120 also features extensive memory and battery life for efficient, extended monitoring, dual power capabilities, flexible print modes, automated NIBP readings, and simple on-site calibration.
April 2008


 October 21, 2025
October 21, 2025