March 10, 2008 - Royal Philips Electronics last week unveiled the HD7, the latest addition to its HD ultrasound family ...
Nuance Communications Inc.'s on-demand offering for critical test result management (CTRM), Vocada Veriphy 2.0 is an end-to-end enterprise solution for communicating critical test results from hospital diagnostic departments to ordering clinicians.
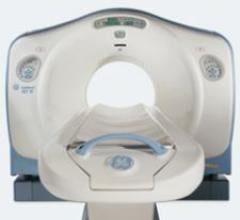
March 10, 2008 - The FDA gave Siemens 510(k) market clearance of four new software applications used with the SOMATOM Definition aimed at simplifying the diagnosis of diseases of the heart, brain, lungs and extremity joints, as announced at ECR 2008, currently being held in Vienna.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
March 7, 2008 - Siemens Healthcare launched the DCA Vantage Analyzer, a point-of-care (POC) diabetes patient management ...
GE's SnapShot Pulse offers improved dose reduction results for both diagnostic cardiac and neuro perfusion CT exams and ...
March 7, 2008 – KLAS recently named Toshiba’s Aquilion 64, Xario and Vantage 1.5T MR “Best in KLAS” in its Top 20 Year ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Siemens Healthcare now has available the DCA Vantage Analyzer, a point-of-care (POC) diabetes patient management ...
March 7, 2008 – CircuLite has advanced its Synergy Pocket Circulatory Assist Device clinical program into a 20-patient trial, after completion of a first-in-man pilot study of Synergy in four patients, as the company seeks CE Mark approval in the European Union for long-term implantation of Synergy in heart failure patients.
March 6, 2008 - Axellis Ltd., the UK- and US-based medical technology company, has completed acquisitions of three ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
March 5, 2008 - The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Evaluation Agency ...
March 6, 2008 - The third annual Medical Device Regulatory, Reimbursement and Compliance Congress (Device Congress) to ...
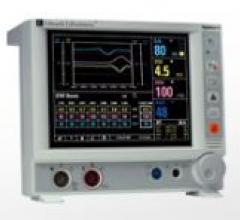
Edwards Lifesciences provides The Vigilance II monitor, which is designed to gives users the ability to present complex ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
February 5, 2008 - Doctors completed the first implant of Boston Scientific’s COGNIS cardiac resynchronization therapy defibrillator (CRT-D), which reportedly represents an entirely new platform to treat heart failure.
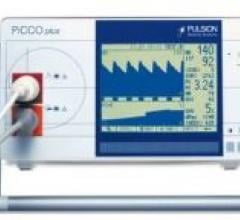
For patient monitoring systems not integrated with PiCCO-Technology, Pulsion Medical Systems offers the PiCCO parameters ...
March 3, 2008 - Boston Scientific Corp. received CE Mark approval of its ACUITY Spiral left ventricular lead for use with cardiac resynchronization therapy defibrillators and cardiac resynchronization therapy pacemakers, marking the company�s seventh U.S. or European regulatory approval in the past nine weeks.

 March 09, 2008
March 09, 2008