June 11, 2025 — MorningStar Laboratories, LLC, a developer of precision diagnostic tests that address unmet clinical ...
June 04, 2025 — HeartSciences Inc. has announced that the U.S. Food and Drug Administration has granted Breakthrough ...
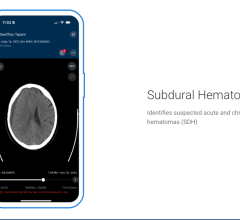
June 12, 2025 — Viz.ai recently announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
June 12, 2025 – The American Society of Echocardiography (ASE) and Cardiovascular Research Foundation (CRF) have ...
June 12, 2025 — GE HealthCare has announced the combination of GE HealthCare’s proprietary features and algorithms with ...
June 11, 2025 — Bayer and the Broad Institute have have extended their research collaboration of 10 years by an ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

As healthcare systems continue to shift toward value-based care and proactive disease management, remote monitoring has ...
June 6, 2025 – A large retrospective review of more than 100,000 patients found the use of glucagon-like peptide-1 ...
June 5, 2025 – Penumbra, Inc. has announced the U.S. Food and Drug Administration (FDA) clearance and launch of the Ruby ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
June 4, 2025 — A new study published in The Annals of Thoracic Surgery, a journal from The Society of Thoracic Surgeons ...
June 3, 2025 — Bayer announced it will present multiple new analyses of the Kerendia (finerenone) clinical trial program ...
May 30, 2025 — BiVACOR, a clinical-stage medical device company developing the world’s first titanium Total Artificial ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
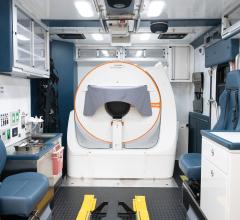
May 28, 2025 — Siemens Healthineers has introduced the first mobile stroke unit (MSU) featuring the Somatom On.site head ...
May 27, 2025 — Abbott has announced the U.S. Food and Drug Administration (FDA) has approved the company's Tendyne ...
May 27, 2025 — CeleCor Therapeutics has completed its multinational Phase 3 clinical trial of DisaggproT (zalunfiban) ...


 June 16, 2025
June 16, 2025