March 16, 2012 — Epocrates is releasing two new, free mobile device applications for cardiologists during the American College of Cardiology 2012 meeting.
March 16, 2012 - Stroke survivors who like art have a significantly higher quality of life than those who do not, according to new research. Patients who appreciated music, painting and theatre recovered better from their stroke than patients who did not.
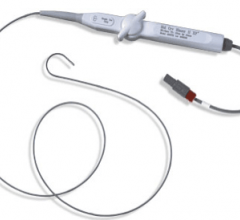
March 16, 2012 — Boston Scientific Corp. announced Health Canada approval of the Blazer open-irrigated catheter, the company's latest radiofrequency ablation (RFA) catheter designed to treat a variety of arrhythmias such as atrial fibrillation (AF), atrial flutter, ventricular tachycardia and other supraventricular tachycardias.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
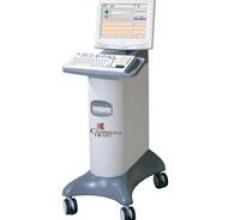
March 16, 2012 – Medistim’s VeriQ system, which uses ultrasound to assess graft blood flow during coronary artery bypass graft (CABG) surgery, recently withstood the clinical scrutiny of the English National Health Service. The VeriQ system combines ultrasound imaging and proven transit time flow measurement (TTFM) in a single system that is specifically designed for cardiovascular procedures.
March 16, 2012 - Depression increases the risk of death in patients who have a coronary stent implanted. After seven years of follow up, depressed patients were 1.5 times more likely to have died than non-depressed patients. The findings were independent of age, gender, clinical characteristics, anxiety and the distressed (Type D) personality.
March 16, 2012 - Cambridge Heart Inc., a developer of non-invasive diagnostic tests for cardiac disease, announced that its Microvolt T-Wave Alternans (MTWA) test received reimbursement coverage from Japan’s Ministry of Health, Labor and Welfare (MHLW). Effective April 1, 2012, the MTWA Test will be reimbursed for patients who are considered at risk for lethal arrhythmias including, but not limited to, patients with a history of heart attack, cardiomyopathy and Brugada syndrome.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
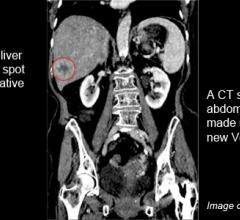
March 16, 2012 - A technological breakthrough is allowing the University of Michigan Health System to be the first teaching hospital in the United States to perform some CT scans using a fraction of the radiation dose required for a conventional CT image.
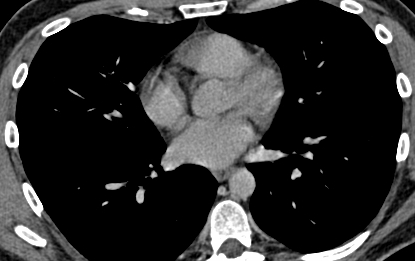
The Heart Rhythm Society (HRS) issued an international consensus statement on indications, techniques and outcomes of catheter and surgical ablation of atrial fibrillation (AF).
March 15, 2012 — According to Millennium Research Group (MRG) the interventional cardiology (IC) market in Pakistan, Saudi Arabia, South Africa and Turkey will reach a value of more than $460 million by 2016.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
March 13, 2012 — A new study by Brigham and Women’s Hospital (BWH) researchers found significant variation in the use of head computed tomography (CT) exams among doctors within the emergency department (ED).
March 20, 2012 - TyRx Inc., a company specializing in the commercialization of implantable medical devices designed to help reduce surgical-site infections, announced the launch of its new patient education website, HeartDeviceInfection.com.
March 13, 2012 — Asahi Kasei Corp. and Zoll Medical Corp. jointly announced that Asahi Kasei will acquire Zoll for approximately $2.21 billion.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
March 12, 2012 — Philips introduced the tactically enhanced HeartStart MRx monitor-defibrillator for emergency medical services (EMS).
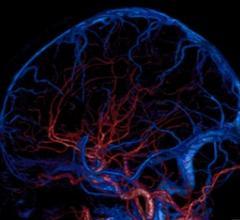
March 12, 2012 — A new study shows that the use of gadobenate dimeglumine, the highest relaxivity gadolinium-based contrast agent (GBCA) available for central nervous system (CNS) magnetic resonance imaging (MRI), over a high concentration MRI contrast agent may improve clinicians’ ability to visualize lesions of the brain.
March 12, 2012 — Siemens Healthcare announced shipment of the Acuson S1000 ultrasound system, a premium ultrasound platform. The Acuson S1000 system expands access to imaging performance at a very low cost of ownership.

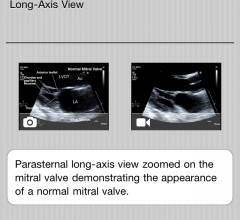
 March 16, 2012
March 16, 2012