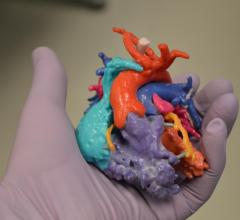
A new 3-D computer modeling system may significantly improve a surgeon’s ability to select the best-sized donor heart for children receiving heart transplants, according to research presented at the American Heart Association’s Scientific Sessions 2015.
NaviGate Cardiac Structures Inc. (NCSI) announced that a first-in-human implant of its catheter-guided, mitral-valved stent into a beating heart was performed successfully in a 53-year-old male patient presenting with severe 4+ mitral regurgitation (MR).
CIRS ATOM phantoms are a full line of anthropomorphic, cross sectional dosimetry phantoms designed to investigate organ ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Boston Heart Diagnostics announced the launch of StatinSmart, an at-home saliva laboratory developed test that analyzes the SLCO1B1 (Solute Carrier Organic Anion Transporter 1B1) gene for a variant known to increase an individual's risk for developing statin-induced myopathy.
Boston Scientific announced the first implants of the Watchman FLX left atrial appendage closure (LAAC) device
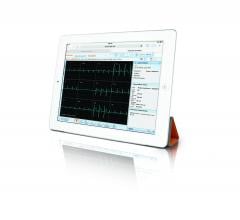
Carestream has obtained U.S. Food and Drug Administration (FDA) clearance for diagnostic reading of electrocardiogram (ECG) waveforms on desktop displays and mobile tablets using its Vue Motion universal viewer.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
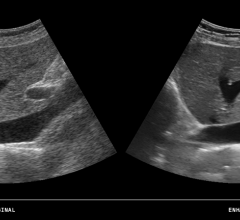
At this year’s scientific assembly and annual meeting of the Radiological Society of North America (RSNA), ContextVision will be demonstrating its wide range of leading 2-D/3-D/4-D imaging enhancement software solutions for ultrasound.
Rijuven announced the launch of CardioSleeve for Pediatrics, the first U.S. Food and Drug Administration (FDA)-cleared device that adds electrocardiogram (ECG) capabilities to transform any existing stethoscope into a smart, mobile-connected device.
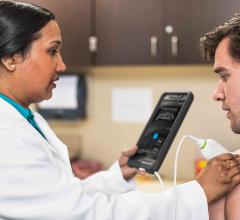
Philips announced that Lumify, a smart device ultrasound solution, is available for purchase by licensed healthcare providers or organizations in the United States.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
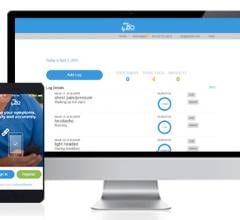
iRhythm Technologies Inc. announced the launch of new patient engagement tools to enhance the diagnosis of cardiac arrhythmias. Patients using the ZIO continuous cardiac monitoring service from iRhythm can now conveniently log heart symptoms with the new myZIO app for iPhone or at myZIO.com.
Healthcare research firm KLAS released its annual rankings of the best-performing medical equipment vendors. The 2015 ...
Fujifilm SonoSite Inc. announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for iViz, its newest ultrasound medical visualization solution. First commercialized in Europe, iViz was developed from the ground up to meet the needs of the highly-mobile clinician.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Konica Minolta Inc. announced that it has developed the SPFS Immunoassay System, capable of highly sensitive measurements based on the fluorescent antibody technique. A prototype will be on reference exhibit at MEDICA 2015, the world’s largest international medical equipment fair in Dusseldorf, Germany, Nov. 16-19, 2015.
Today, magnetic resonance imaging (MRI) allows more gentle, precise and cost-effective heart disease diagnosis. However, the method has limitations when examining children and patients with cardiac arrhythmia. A joint project of the Fraunhofer Institute for Medical Image Computing MEVIS and the Max Planck Institute for Biophysical Chemistry promises a solution for these limitations. Experts were able to accelerate the image acquisition process and expand the method’s applicability.

There is a growing need to exchange and share imaging studies with outside entities. Hospitals that have foregone the ...

 November 23, 2015
November 23, 2015