Barco announces the launch of its new Eonis clinical display family. Designed with clinical specialists in mind, the Eonis displays combine an innovative, built-in front sensor with Barco’s MediCal QAWeb cloud-based tool to ensure superior, controlled image quality. The Eonis 22-inch model is available in a black and a white version, with the latter offering a fully cleanable front glass panel to prevent infection – a first in the market.
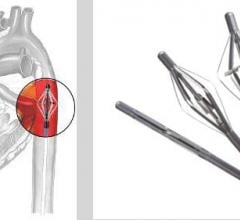
Reva Medical Inc. announced that it has initiated patient enrollment with its ReZolve2 bioresorbable sirolimus-eluting coronary scaffold.
Lantheus Medical Imaging Inc. announced a new supply contract with the Institute for Radioelements (IRE) to receive a supply of molybdenum-99 (Mo-99), the parent isotope of technetium-99m (Tc-99m), for use in its TechneLite (Technetium Tc 99m Generator) generators.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Two new 1.5T MRI scanners from GE Healthcare, the Optima MR360 Advance and Brivo MR355 Inspire, have recently received 510(k) clearance. Redesigned with new enclosures symbolizing GE’s Humanizing MRI strategy, both systems are engineered to address the demand for increased performance and reduced total cost of ownership for the facility, while providing a comfortable experience for the patient.
The American Society of Echocardiography (ASE) has released a list of five interventions whose appropriateness physicians and patients should discuss as part of Choosing Wisely, an initiative of the ABIM Foundation, along with Consumer Reports. Fourth in the list, they ask that patients and their doctors talk about the real need for a stress echocardiogram if they do not present conditions that would warn them of a risk of heart disease.
Rox Medical announced enrollment of the first U.K. patients in the CONTROL-HTN international randomized controlled trial of the Rox Flow procedure for the treatment of resistant hypertension.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

This is a roundup of some of the DAIC editor's choice of the most innovative new technologies showcased at ACC 2013, including products in the areas of interventional cardiology, patient monitoring, electrophysiology and echocardiography.
Cardiologists at ACC.13 learned that patients with coronary artery disease who received a Resolute drug-eluting stent from Medtronic Inc. as participants in one of several clinical studies and interrupted or discontinued their dual antiplatelet therapy after one month of the implant procedure showed no increased safety risk through one year of follow-up.
Thoratec Corp. announced that it has successfully completed the first human use of HeartMate PHP (percutaneous heart pump). The first PHP patient was supported for over 60 minutes during a high-risk percutaneous coronary intervention (HR PCI). The patient was hemodynamically stable during the procedure, which involved three-vessel intervention on a patient with an ejection fraction less than 30 percent. Two additional patients were treated as part of this first-in-man series. The procedures were performed by Adrian Ebner, M.D. at Sanatorio Italiano in Asuncion, Paraguay. Ebner is the chief of the Cardiovascular Department at Sanatorio Italiano.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
St. Jude Medical announced publication of results from its landmark RESPECT clinical trial in The New England Journal of Medicine.
Minimally invasive procedures significantly lower health payer costs and result in fewer missed workdays when compared to open surgery, according to a study published this week in the Journal of the American Medical Association (JAMA) Surgery. Of six procedures examined, the percutaneous coronary intervention (PCI) arm of the study drove the cost savings. This is an important finding, considering that heart disease remains the leading cause of death and disability in the United States and accounts for considerable expenditures in healthcare services, medications and lost productivity.
A new test that detects asymptomatic individuals who may be at risk for cardiovascular disease is being launched in Europe at the Healthcare Innovation Expo 2013. The device, known as AngioDefender, was developed by Everist Genomics Inc. of Ann Arbor, Mich., in collaboration with scientists at Rutgers University, New Jersey. The developers claim that the test will lower the cost of detecting and treating cardiovascular problems and improve patient outcomes.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

Biotronik received CE mark for its most advanced ICD/CRT-D (implantable cardioverter defibrillator/cardiac resynchronization therapy device) series. Iforia is the world’s first DF4 ICD/CRT-D series approved for MRI (magnetic resonance imaging). In addition it contains one of the world’s smallest ICDs, the Iforia single chamber ICD.
The Heart Failure 2013 LA meeting will include a showcase of several startup companies offering innovative new approaches to address heart and cardiovascular disease.
The U.S. Food and Drug Administration (FDA) notified healthcare professionals of a Class I recall of the Vascular Solutions Inc. Guardian II and Guardian II NC Hemostasis Valves. The firm is recalling the product due to a risk that air may be introduced into the device which may lead to an air embolism. This product may cause serious adverse health consequences, including death.

 April 02, 2013
April 02, 2013