September 5, 2008 - The results of the SYNTAX study, a head-to-head comparison between drug-eluting stents (DES) using ...
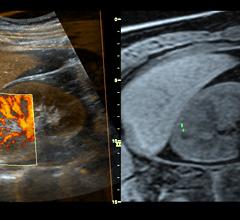
September 5, 2008 - GE Healthcare today said it launched a new ultrasound system for radiology and vascular applications, the LOGIQ E9, which fuses ultrasound images with images from other imaging modalities like CT and MR.
Biotronik GmbH launched the Lumax 540 series, which includes implantable cardioverter defibrillators (ICDs) and a cardiac resynchronization therapy defibrillator (CRT-D), which is used with Biotronik Home Monitoring to enable continuous automatic daily data transmission of the patient’s cardiovascular status via the Internet.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
September 2, 2008 - GE Healthcare launched a new ultrasound system for radiology and vascular applications that fuses ...
September 5, 2008 - Pathway Medical Technologies Inc., today received FDA clearance to market Jetstream, a peripheral atherectomy catheter designed for use in the treatment of peripheral artery disease (PAD) in the lower limbs.
September 5, 2008 - Boston’s Brigham & Women’s (B&W) Hospital, a 777-bed teaching affiliate of Harvard Medical School ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Vascular Solutions will partner with Radius Medical Technologies for the U.S. launch of the MICRO Elite Snare, a device ...
Boston Scientific launched its Atlantis 018 Peripheral Imaging Catheter, which reportedly brings existing 40MHz coronary ...
The FDA has approved the LipiScan Coronary Imaging System, an angiography laser scanner by InfraReDx Inc., which is intended to characterize fatty deposits in coronary vessel walls using near infrared diffuse spectroscopy.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The recently FDA-approved COGNIS CRT-D and the TELIGEN ICD from Boston Scientific are among the smallest and thinnest high-energy devices at 32.5 cc and 31.5 cc respectively, while less than 10 mm thick. Both devices offer extended battery longevity over previous company devices, self-correcting software and improved programming technology.
September 3, 2008 - CardioNet Inc. said today Humana upgraded the CardioNet System to a “covered benefit” status, from ...
While the primary reason hospitals adopt new technology is to increase patient care and produce better outcomes, there are many other factors that also affect purchasing decisions.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Spectranetics Corp. recently announced the availability of its LLD EZ Lead Locking Device (LLD) for the removal of ...
TRUMPF Medical Systems Inc. offers an in-light high-definition (HD) operating room camera, the TruVidia HD, which ...
St. Jude Medical’s Promote RF CRT-D (cardiac resynchronization therapy defibrillator) and Current RF ICD (implantable cardioverter defibrillator) are radiofrequency (RF) wireless devices used to treat patients with heart failure and with potentially lethal heart arrhythmias.

 September 04, 2008
September 04, 2008