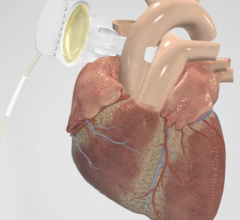
Devices like pacemakers and defibrillators allow doctors to introduce electrical signals to set patients' hearts at regularly timed beats. However, these small mechanical devices come with risks.
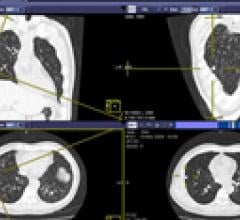
Infinitt will introduce its new “Communicator” package at RSNA 2013. It is an add-on that ensures immediate communication of critical test results, provides peer review and emergency room discrepancy management and includes an embedded communication feature that allows real-time collaboration between users of the Infinitt picture archive and communications system (PACS). This is particularly valuable for clarification between the study’s dictating physician and transcriptionist or the referring physician.

Early treatment of heart attack patients with an inexpensive beta-blocker drug called metoprolol, while in transit to the hospital, can significantly reduce damage to the heart during a myocardial infarction, according to clinical trial/study results published Oct. 1 in the journal Circulation. The study was a collaboration between Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC) and Icahn School of Medicine at Mount Sinai.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

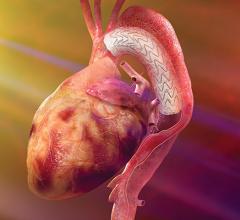
As a cutting-edge technique, many hospitals around the country are investigating the development of their own TAVR programs. Starting a TAVR program requires a collaborative commitment to care and advanced imaging technology to plan and perform the exams. With the right approach and equipment, a TAVR program can have a profound impact on patients and the hospitals that serve them.
Sudden cardiac death (SCD) is the leading cause of death in high school-aged athletes. To determine if SCD can be prevented with a heart screening, The Christ Hospital Health Network (Cincinnati, Ohio), The University of Mississippi Medical Center and Cincinnati Children’s Hospital Medical Center partnered with USRowing and Toshiba America Medical Systems Inc. to conduct The Athlete Heart Research Study. Initial participants included volunteer high school rowers with more than two years of continuous practice who competed in the USRowing Youth National Championships, June 7 – 9, 2013, in Oak Ridge, Tenn.

The U.S. Food and Drug Administration (FDA) released a draft of its proposed updates for the non-clinical engineering tests and recommended labeling for intravascular stents and associated delivery systems. It is designed to provide draft guidance for industry and FDA staff.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Methodist Le Bonheur Healthcare (MLH) will deploy McKesson’s portfolio of enterprise medical imaging products across its facilities to improve physician collaboration, streamline data management and simplify its imaging archive process with a single point of access.
Rochelle Community Hospital purchased and installed Carestream’s Vue Cardio PACS (picture archive and communications system) that provides fully featured viewing and management for its echo cardiograms and nuclear cardiology exams. This versatile system will also support ECGs and other types of cardiac exams that the critical access hospital may add in the future.
The U.S. Food and Drug Administration (FDA) approved Biotronik’s Ilesto family of implantable cardioverter-defibrillator/cardiac resynchronization therapy defibrillators (ICD/CRT-D devices). Biotronik follows this with the launch of its next generation technology platform Ilesto DX.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The U.S. Food and Drug Administration (FDA) approved the Conformable Gore TAG Thoracic Endoprosthesis for endovascular repair of acute and chronic Type B dissections of the descending thoracic aorta. The Conformable Gore TAG Thoracic Endoprosthesis is designed for multiple thoracic etiologies, and is the only device to receive FDA indications for aneurysm, trauma and dissection. Previously, the only approved treatment options were medical management or open surgical repair.
Expanding its role in the treatment of peripheral artery disease in the United States, Medtronic Inc. announced that the U.S. Food and Drug Administration (FDA) has approved the Complete SE (self-expanding) vascular stent for use in the lower extremities –– specifically, the superficial femoral artery (SFA) and proximal popliteal artery (PPA), which carry blood through the upper legs.
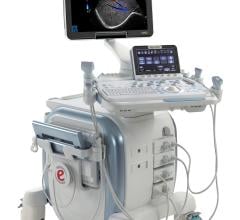
Clinicians face the challenge of effectively imaging larger patients. Recognizing this trend, Esaote’s new eHD Technology improves every element of the imaging chain and increases ultrasound’s ability to image with more clarity at greater depth.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The Cardiovascular Research Foundation (CRF) announced the late breaking trials and first report investigations that will be presented at the Transcatheter Cardiovascular Therapeutics (TCT) 2013 scientific symposium in late October.
Whole-body magnetic resonance imaging (MRI) may serve as a valuable noninvasive tool for assessing the risk of heart attack and stroke in diabetic patients, according to a new clinical study published online in the journal Radiology.
Sunshine Heart Inc. has launched a website dedicated to providing information to patients suffering from moderate to severe heart failure to learn about its C-Pulse U.S. pivotal trial, COUNTER HF(TM).

 October 03, 2013
October 03, 2013