September 24, 2012 — Bio DG announced that its patent for a novel metal system, to use as biodegradable material in developing implantable medical devices, was granted by the U.S. Patent Office on Aug. 21, 2012. Bio DG's biodegradable alloys are primarily metallic and provide high strength when first implanted, then gradually erode in a predictable and biocompatible manner.
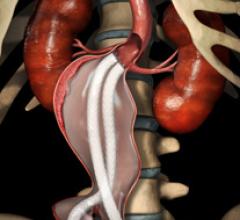
September 21, 2012 — Endologix Inc. announced this month it received CE mark for the current version of the Nellix EndoVascular Aneurysm Sealing System for the treatment of patients with abdominal aortic aneurysms (AAA).
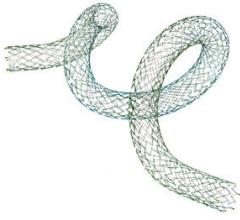
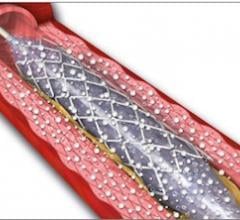
September 21, 2012 — Following the success of Biotronik’s Pulsar-18 0.018-inch, 4 French platform self-expanding stent system, the company has introduced its Pulsar stent technology in a 0.035-inch, 6 French platform.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
September 21, 2012 — Cardionovum GmbH announced that the results of a preclinical study and a first-in-man clinical study of its Primus drug-coated balloon (DCB) will be published in the October issue of Minerva Cardioangiologica, the journal of the Italian Society of Angiology and Vascular Pathology and of the Italian Society of Vascular Diagnostics.
September 20, 2012 — Hospitals, medical facilities, doctors and long-term care facilities this fall are stocking shelves and purchasing items from their wish list and budgets before a new tax on most medical products goes into effect in 2013.
September 20, 2012 — Boston Scientific Corp. has signed a definitive agreement to acquire BridgePoint Medical Inc., a privately held company based in Minneapolis. BridgePoint Medical has developed a proprietary, catheter-based system to treat coronary chronic total occlusions (CTOs).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
September 19, 2012 — Heart catheter procedures guided by magnetic resonance imaging (MRI) are as safe as X-ray angiography-guided procedures and take no more time, according to a pilot study conducted at the National Institutes of Health (NIH). The results of the study indicate that real-time MRI-guided catheterization could be a radiation-free alternative to certain X-ray-guided procedures.
September 19, 2012 — Merge Healthcare Inc. announced that its board of directors has retained Allen & Company LLC, a New York-based investment bank, to assist in exploring and evaluating a broad range of strategic alternatives including, but not limited to, a sale of the company or a business combination.
September 19, 2012 — This past week, surgeons and cardiologists at Sentara Heart Hospital were the first in the world to begin performing surgeries in the Dual Epicardial Endocardial Persistent Atrial Fibrillation (AF) Study (Staged DEEP) feasibility trial.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
September 19, 2012 — In response to criticism by physicians about the restrictiveness of conditions put forth by the Center for Medicare and Medicaid Services (CMS) for reimbursement for transcatheter aortic valve replacement (TAVR), the American Society of Echocardiography (ASE) is emphasizing the importance of heart team quality and experience in ensuring positive patient outcomes.
First-year medical students at Mount Sinai School of Medicine will be the first in New York to be introduced to a digital-age ultrasound device that can visualize inside the body, and fit directly into the pockets of their brand new white coats.
September 18, 2012 — Inpatient hospital treatment accounts for the largest proportion of healthcare spending in the United States, with the use of diagnostic imaging services such as magnetic resonance images (MRIs) frequently implicated as the probable cause. A new analysis finds that the biggest expense may not be from imaging technology, but from supplies including medical devices, such as stents and artificial joints.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
September 18, 2012 — Neovasc Inc. announced that acute results from preclinical studies of its Tiara valve for the transcatheter treatment of mitral regurgitation were published in the Journal of the American College of Cardiology (JACC). The study reports that the initial experience with the Tiara transcatheter mitral valve was encouraging, and that implantation of Tiara valves was feasible, relatively straightforward and resulted in a securely-implanted, well-functioning device that maintained good hemodynamics in the test animals. The study, which will be published in the Oct. 9, 2012, edition of JACC, is currently available online.
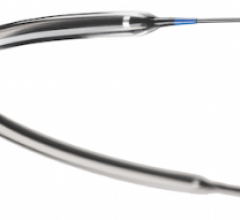
Covidien announced the launch of the Viance Crossing Catheter and Enteer Re-entry System for treating chronic total occlusions (CTOs) endovascularly. The devices are now available in the United States, the European Union (EU) and other select international markets.
September 18, 2012 — Abiomed Inc. announced it received confirmation from the American Medical Association (AMA) of three new Category I Current Procedure Terminology (CPT) codes for Impella percutaneous technologies, effective January 2013.

 September 24, 2012
September 24, 2012