April 23, 2014 — Xeltis announced it finished enrollment in a five-patient feasibility study of implantable products intended to enable for the first time the spontaneous growth of natural, healthy heart valves and vessels.
April 23, 2014 — Philips Healthcare has completed its first field study for its Minicare handheld cardiac Troponin-I (cTn-I) blood test, demonstrating the platform’s potential to produce lab-equivalent results with finger prick samples within minutes. The initial results are encouraging as Philips prepares for the Minicare cTn-I system’s clinical trial, scheduled for 2015.
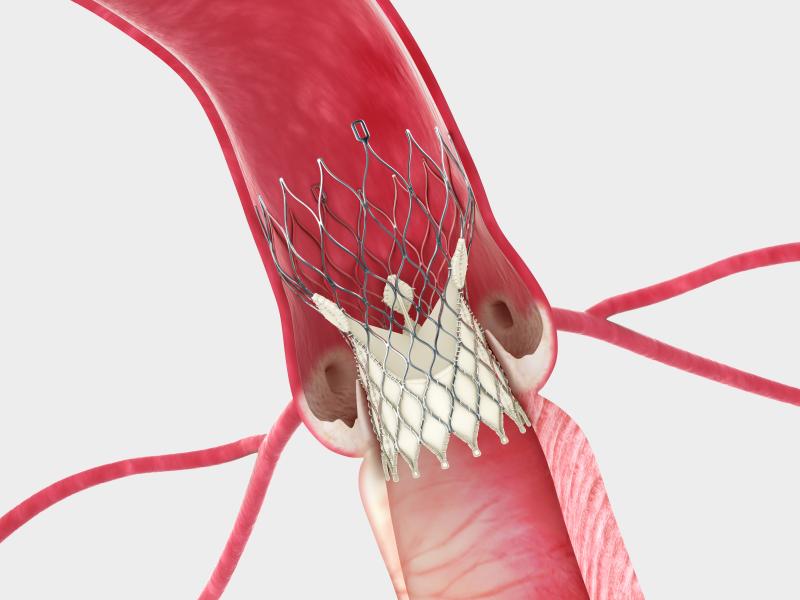
The Federal Circuit Court of Appeals granted a request from Medtronic Inc. to postpone the implementation of an injunction that would have prevented the company from selling its CoreValve System in the United States. The April 21 decision means the injunction will only take effect if the appellate court determines the injunction was properly issued.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Loyola University Medical Center is now offering patients the most advanced positron emission tomography/computed tomography (PET/CT) scanner on the market.
Volcano Corp. announced U.S. Food and Drug Administration (FDA) clearance of its proprietary Instant Wave-Free Ratio (iFR) modality.
April 21, 2014 — Decision Resources Group announced in a report that Servier's ivabradine (procoralan) is set to become the clinical gold standard in the treatment of chronic heart failure (CHF). Already available in Europe, where it has enjoyed moderate use in CHF and stable angina patients, ivabradine's clinical profile could prime this agent for a potentially successful launch in the United States, where Amgen own the marketing rights to procoralan.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
April 21, 2014 — Cardiologists and cancer experts at the Mount Sinai Hospital in Manhattan have joined forces to establish its first cardio-oncology clinic at the Tisch Cancer Institute.
Providence Saint Joseph Medical Center in Burbank, Calif., is the first hospital in the nation to conduct a magnetic resonance imaging (MRI) scan of a patient implanted with a new MRI-compatible pacemaker — a breakthrough because metal implants often exclude patients from this imaging because of the strong magnetic force.
Treatment options for high-risk heart patients with severely calcified coronary artery disease (CAD) have been limited for more than 20 years. Now, Robert Wood Johnson University Hospital (RWJUH) in New Brunswick, N.J., offers a new alternative to open up blocked arteries.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Tendyne Holdings Inc. has secured $25 million in an oversubscribed Series C financing led by Apple Tree Partners, along with Boulle Group members and other existing investors.

Cytori Therapeutics announced publication of safety and efficacy data from a 36 month European clinical trial of Cytori Cell Therapy in patients with chronic ischemic heart failure.
April 18, 2014 — New research presented at the 34th annual meeting of the International Society for Heart and Lung Transplantation (ISHLT) demonstrated the CorMatrix particulate extracellular matrix (P-ECM) alone and as an adjunct to HeartWare International Inc.’s left ventricular assist device (LVAD) improved cardiac function in a bovine ischemic heart failure model, as compared to LVAD alone.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
April 18, 2014 — A University of California, San Diego scientist has developed a new biomaterial that could potentially treat heart attacks. Karen Christman, Ph.D., associate professor of bioengineering at UC San Diego Jacobs School of Engineering, has been at work on a new injectable hydrogel, which is designed to repair damaged cardiac tissue following a heart attack.

April 18, 2014 — AliveCor announced the online publication of study results of the SEARCH-AF study. The SEARCH-AF study, conducted in Australia, screened 1,000 customers over 65 years old for atrial fibrillation (AF) in 10 community pharmacies in suburban Sydney.
Medtronic announced the U.S. Food and Drug Administration (FDA) approved an expanded indication for biventricular (BiV) pacing with Medtronic cardiac resynchronization therapy-pacemakers and -defibrillators (CRT-P and CRT-D). Medtronic CRT devices are now approved to treat patients with atrioventricular (AV) block and left ventricular (LV) systolic dysfunction, reducing heart failure hospitalizations and mortality, and improving cardiac function in these patients.


 April 23, 2014
April 23, 2014








