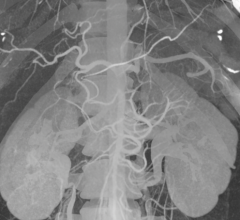
Imris Inc. announced that a University of South Florida neurosurgical team at Tampa General Hospital (TGH) completed initial cases using its recently installed Visius intraoperative MRI (magnetic resonance imaging) — providing the most advanced surgery and operating suite in the Tampa Bay area.
April 30, 2014 — Fovia Medical Inc. and Imperial College London, a science-based institution in biomedical research, announced a collaboration to bring High Definition Volume Rendering (HDVR) to minimally invasive robotic surgery.
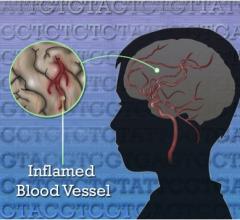
National Institutes of Health (NIH) researchers have identified gene variants that cause a rare syndrome of sporadic fevers, skin rashes and recurring strokes, beginning early in childhood.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Scripps Health became the first health care provider in Southern California to implant the investigational Nanostim leadless pacemaker, the world's first wireless pacemaker totally implanted in the heart, as part of the Leadless II Clinical Trial.
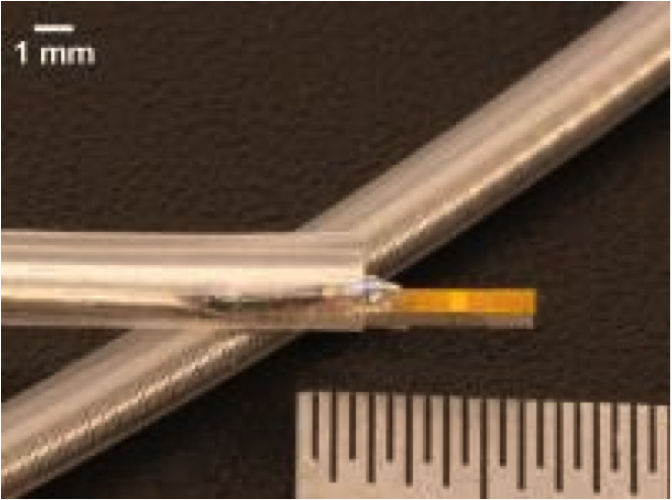
April 30, 2014 — Researchers from North Carolina State University and the University of North Carolina at Chapel Hill have developed a new ultrasound device that could help identify arterial plaque that is at high risk of breaking off and causing heart attack or stroke.
April 30, 2014 — Celladon Corp. announced its lead product candidate, Mydicar, has been granted breakthrough therapy designation by the U.S. Food and Drug Administration (FDA) for reducing hospitalizations for heart failure in NYHA class III or IV chronic heart failure patients who are NAb negative. Celladon is a clinical-stage biotechnology company developing novel therapies for patients with heart failure and other diseases characterized by SERCA enzyme deficiencies.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
April 29, 2014 — Armetheon Inc. announced it has reached agreement with the U.S. Food and Drug Administration (FDA) on a special protocol assessment (SPA) for the final pivotal trial of tecarfarin. Tecarfarin is positioned to be the only oral anticoagulant therapy for patients with prosthetic heart valves (PHV) specifically identified in the label.
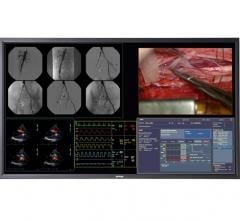
Healthcare imaging specialist Barco launched its MDSC-8258, a 58-inch surgical display designed for 4K imaging in hybrid operating rooms and interventional suites.
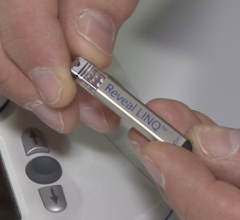
John Muir Health announced that its Concord, Calif., medical center is one of the first hospitals in Northern California to implant a miniature cardiac monitor in a patient – the Reveal Linq Insertable Cardiac Monitor (ICM) System.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
When an unmanned supply mission launched into space on April 18, bound for the International Space Station, it meant something extraordinary to Peter Lee, a cardiothoracic surgeon at The Ohio State University Wexner Medical Center. That is because his research experiment is on board.
April 28, 2014 — JenaValve Technology Inc. – a privately held, venture-backed developer, manufacturer and marketer of transcatheter aortic valve implantation (TAVI) systems – announced it has secured an expansion to its Series C venture round of $10 million, increasing the total amount raised in the round from $62.5 million to $72.5 million.
April 28, 2014 — John C. Lincoln North Mountain Hospital in Phoenix is the first in Arizona, and only one of eight hospitals in the United States, to reduce or eliminate radiation for minimally invasive cardiac procedures utilizing MediGuide technology.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
April 28, 2014 — Boston Scientific Corp. has conducted the first implant in the clinical trial of the next-generation Acuity X4 left ventricular (LV) pacing leads and Reliance 4-Front implantable converter defibrillators (ICD) leads. The clinical trial is designed to establish the safety and effectiveness of both lead families and is intended to support U.S. Food and Drug Administration (FDA) approval of these devices.
Shina Systems announced the new commercial release of its 3Di PACS (picture archive and communications) solution.
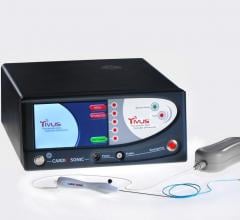
Cardiosonic Ltd. announced CE marking of its TIVUS (therapeutic intravascular ultrasound) ablative catheter device.

 April 30, 2014
April 30, 2014