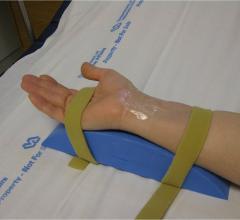
Interventional radiologists have devised a way to access a woman's fibroids by flipping her wrist and treating via an arm not groin artery. It can be used to nonsurgically shrink noncancerous growths in the muscular wall of the uterus. Researchers found this to be less painful and traumatic for women, allowing them to immediately sit up and move after uterine fibroid embolization (UFE)—with no overnight stay. This is according to a March article in the Journal of Vascular and Interventional Radiology.
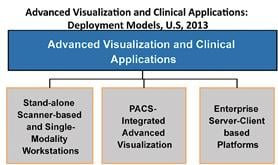
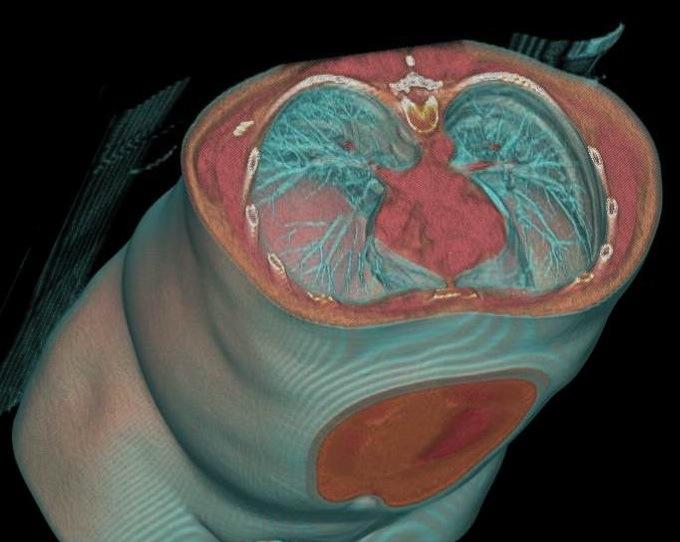
If the exhibit floor of the 99th Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA) had one standout modality this year, it was, without question, the advances with premium computed tomography (CT). Technological and clinical advances in magnetic resonance (MR) and molecular imaging were less impressive but were no less significant. Naturally, these developments in every advanced imaging modality are turning eyes to advanced visualization (AV) and clinical applications. AV is not just a core step in the interpretation workflow of each of these modalities. More than that, it is the set of solutions that will allow the realization of the promises of these imaging advances — the ones that will “make sense” of these upgraded imaging capabilities by using innovative ways to interrogate their improved image depth and visualize their enhanced image quality.
Memorial Hospital in Carbondale, Ill., has chosen Quail Digital’s healthcare wireless headset system. It will be used in catheterization and electrophysiology labs where all coronary and peripheral vascular interventions, device implants and electrophysiology procedures are performed.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
MedStar Heart Institute of Washington, D.C., and Infraredx Inc. enrolled the first participant in the Lipid-Rich Plaque (LRP) Study. The LRP Study is a prospective, multi-center study aimed at identifying a correlation between LRPs and the occurrence of a cardiac event within two years.

Edwards Lifesciences Corp. announced the successful completion of the first three human implants of its Fortis mitral transcatheter heart valve. The Heart Team at St. Thomas’ Hospital in London performed them in February and March.
The Valley Hospital is among a few hospitals in the nation to implant a tiny wireless heart monitor expected to have impacts for patients and doctors.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
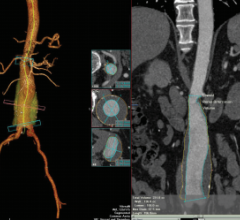
W. L. Gore & Associates’ Gore Excluder Iliac Branch Endoprosthesis is a complete, fully engineered system intended for endovascular treatment of common iliac artery aneurysms or aortoiliac aneurysms. It received CE marking. The first patient procedures in Europe were successfully completed by vascular surgeons Piergiorgio Cao, M.D., chief of vascular surgery at San Camillo Hospital, Rome, Italy, Mo Hamady, M.D., consultant interventional radiologist at St. Mary’s Hospital, London, and Michael Jenkins, consultant vascular surgeon and clinical lead at St. Mary’s Hospital, London.
Despite good immediate results, in up to 40 percent of patients, obstructed arteries in the leg treated with a stent will again become blocked. This in-stent restenosis is typically treated with balloon angioplasty to clear the artery. The addition of drug-eluting balloons is showing improved outcomes for restenosis and could become the treatment of choice for femoropopliteal in-stent restenosis.
Biotronik announced the first implantations of the Sentus ProMRI lead. Biotronik’s bipolar cardiac resynchronization therapy (CRT) lead is the first MR conditional lead with a 4 French diameter — approximately 1.6 mm. An ultra-thin lead, it enables access to particularly challenging vessels and offers expanded pacing options. In addition, the Sentus leads are allow patients to undergo MRI (magnetic resonance imaging) scans. The CRT lead received CE marking in early February.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The American College of Radiology applauded steps to reign in medical imaging and radiation oncology self-referral included in President Obama’s Fiscal Year 2015 budget. However, prior authorization for imaging services, also included the FY 2015 budget, is unnecessary and will ultimately raise costs, interfere in the doctor-patient relationship and restrict ready access to imaging care.
Blanchard Valley Health System is a non-profit, integrated health system with more than 2,000 associates serving eight counties in Ohio. It selected Merge Healthcare Inc. for its enterprise-wide vendor-neutral archive (VNA), interoperability and cardiology solutions to meet Meaningful Use 2 (MU2) requirements for the exchange of images.
At age 88, Lena M. Smith visited her doctor, Larry Hunt, M.D., at the University of Alabama at Birmingham to find out why her energy level had diminished.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Vital Images Inc., a Toshiba Medical Systems Group Company, is expanding in the EMEA market with an increasing number of organizations migrating to Vital's VitreaAdvanced solution to help improve efficiency, communication and patient care. The VitreaAdvanced advanced visualization solution provides 2-D, 3-D and 4-D images for applications addressing cardiovascular, neurovascular and oncology disease states. Fueled by intelligent automation, it utilizes a clinical workflow to improve speed and simplicity of use.
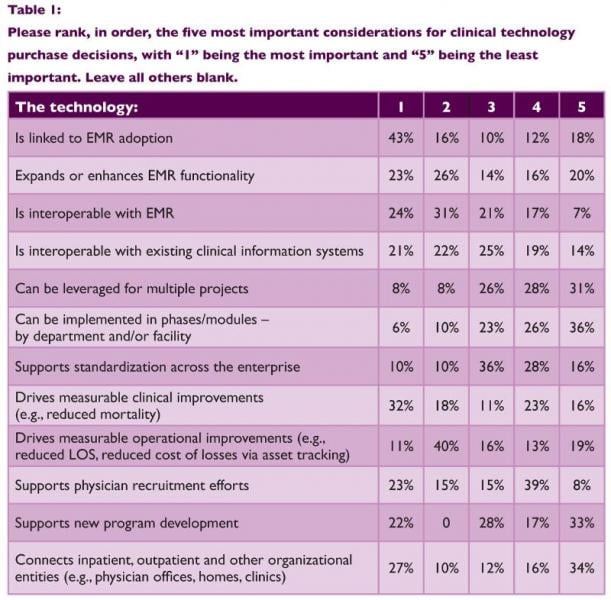
Interoperability, mobile connectivity and technologies that drive real-time actionable information at the point of care will be the focus of health information technology (IT) investments in 2014. This is according to the results of a new healthcare leadership survey released by Philips Healthcare.
Advanced Accelerator Applications, an international specialist in molecular nuclear medicine (MNM), announced it has entered into an agreement to acquire 100 percent of the shares of Imaging Equipment Ltd., a privately held U.K. distributor of nuclear medicine products and technologies.

 March 07, 2014
March 07, 2014