The global sales of drug-eluting balloons (DEBs) across the 10 major markets (10MM) are expected to witness a significant increase over the coming years, from $164m in 2012 to $477m in 2019, at a Compound Annual Growth Rate (CAGR) of 16 percent, following the product’s launch in the United States and Japanese markets, according to a new report from research and consulting firm GlobalData.
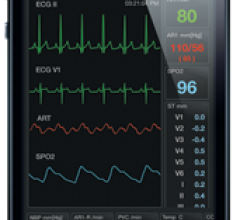
Fluke Biomedical, a provider of medical device test and safety equipment, announced the debut of its new ProSim 2 and ProSim 3 Vital Signs Simulators. Designed for high portability, the lightweight ProSim 2 and 3 are suitable for conducting preventive maintenance and repairs in the field.
Kona Medical Inc. announced it has closed a Series D equity financing of $10 million. Morningside Group, a broad-based investment firm with significant presence in China, led the investment and is the sole investor.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
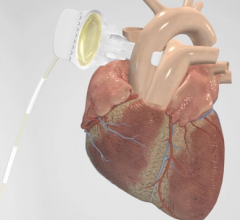
Colibri Heart Valve LLC, a medical device company, announced that patient enrollment has concluded in the first-in-human study of the company's transcatheter aortic valve implantation (TAVI) system in the Dominican Republic trial site.
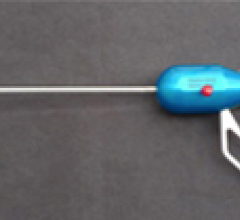
The U.S. Food and Drug Administration (FDA) has granted market clearance for the MitraClip, the first minimally invasive transcatheter device to repair mitral regurgitation (MR). The device is first of its kind to gain approval for use in patients in the United States and part of a new movement away from open heart surgical repair toward minimally invasive procedures performed in a cath lab or hybrid operating room.
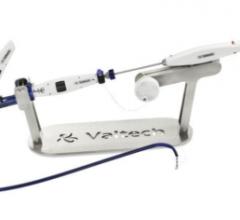
Valtech Cardio Ltd., a medical device company that develops solutions for mitral valve repair and replacement, said 11 patients have been treated with the company's transcatheter (transfemoral) Cardioband Annuloplasty System in three European medical centers.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Transcatheter Technologies GmbH, a medical device company, announced the successful first-in-human implantation of its transapical Trinity aortic valve.

Medical experts released “Guidelines for Performing a Comprehensive Transesophageal Echocardiographic Examination: Recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists.”
AirStrip announced new research demonstrating the results of its mobility solution AirStrip One Cardiology, along with successful use cases by Rockdale Medical Center, Palomar Health, Montefiore Medical Center and Sequoia Hospital.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
PDI, Inc. and Transgenomic, Inc. announced the signing of a U.S. collaboration agreement to commercialize CardioPredict, a molecular diagnostic test developed by Transgenomic. CardioPredict is a broad-based genetic assay which identifies specific genes in patients that influence the effectiveness and safety of many commonly used cardiovascular drugs.
UC Davis Health System researchers have identified for the first time a biological pathway that is activated when blood sugar levels are abnormally high and causes irregular heartbeats, a condition known as cardiac arrhythmia that is linked with heart failure and sudden cardiac death.
Sunshine Heart Inc. announced its collaboration with Minnetronix for a transcutaneous energy transfer (TET) system to power the company's fully-implantable C-Pulse Heart Assist System under development. The agreement leverages Minnetronix's existing TET technology and includes milestone payments due to Minnetronix prior to U.S. regulatory approval for the fully-implantable C-Pulse system.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
In a paper e-published in Catheterization and Cardiovascular Interventions, the Society for Cardiovascular Angiography and Interventions (SCAI) examined the current state of medical simulation in interventional cardiology and issued recommendations for expanding and standardizing the use of the lifelike training technology by practicing interventional cardiologists and fellows-in-training
Cardiovascular Systems Inc. (CSI) announced that it has received premarket approval (PMA) from the U.S. Food and Drug Administration (FDA) to market its Diamondback 360 Coronary Orbital Atherectomy System (OAS) as a treatment for severely calcified coronary arteries.
October 23, 2013 — Vascular Interventional Advances (VIVA) Physicians, a not-for-profit organization dedicated to advancing the field of vascular medicine and intervention through education and research, announced 15 trial results at VIVA 13. The trial results offered new information on advances in the treatment of vascular diseases.

 October 25, 2013
October 25, 2013