Colibri Heart Valve LLC has successfully completed the first clinical use of the company's proprietary transcatheter aortic heart valve. The Colibri transcatheter aortic valve implantation (TAVI) system is the world's first and only low profile, 14 French pre-mounted, pre-crimped, and pre-packaged, ready-for-use, TAVI system. These advantages are designed to reduce delivery profile along with a reduction in preparation and insertion time, which are not available in any other device, either currently available or in known development.
A research study of more than 600 black patients with uncontrolled hypertension found that less than half were prescribed a diuretic drug with proven benefit that costs just pennies a day, report researchers at Weill Cornell Medical College and the Visiting Nurse Service of New York's (VNSNY) Center for Home Care Policy and Research. The researchers say these new findings should be taken as a serious wake-up call for physicians who treat black patients with hypertension.
The Society for Cardiovascular Angiography and Interventions (SCAI) reports on the “Ten Do’s and Don’ts for Improving Documentation in the Catheterization (Cath) Lab” based on studies by the Accreditation for Cardiovascular Excellence (ACE).
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
CardioLogical Solutions, formed by the recent merger of Emboline and VasoStitch, has been issued an additional patent for its family of aortic embolic protection devices that are designed to prevent stroke and other ischemic complications.
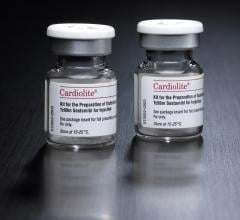
Lantheus Medical Imaging has entered into a new agreement with Fujifilm RI Pharma (FRI) to license and distribute Cardiolite (Kit for the Preparation of Technetium Tc99m Sestamibi for Injection) and Neurolite (Kit for the Preparation of Technetium Tc99m Bicisate for Injection) in Japan.
NeoChord has received CE-mark for its technology that allows the implantation of artificial chordae tendinae, a proven technique for the repair of mitral valve prolapse, via a transapical, off-pump procedure.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
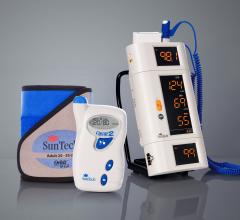
SunTech Medical’s noninvasive blood pressure products have successfully passed multiple interoperability tests during 2013 Integrating the Healthcare Enterprise (IHE) North American Connectathon (Chicago, Ill.) — part of the company’s strategy to develop medical products that can easily share data with different electronic healthcare management (EMR) systems.
ICU Medical Inc. has launched the CardioFlo hemodynamic monitoring sensor system. CardioFlo is a minimally invasive hemodynamic monitoring sensor system that, when used with an existing arterial catheter and connected to an Edwards Lifesciences Vigileo monitor, provides accurate and reliable hemodynamic monitoring data and an average of 40 percent cost savings over the Edwards Lifesciences FloTrac sensor.
Researchers from Boston University School of Medicine (BUSM) have shown that using magnetic resonance imaging (MRI) to measure blood flow over atherosclerotic plaques could help identify plaques at risk for thrombosis. The findings, which appear in the March issue of Circulation Cardiovascular Imaging, offer a non-invasive application in the diagnosis and treatment of patients with atherosclerosis.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Hospital Sisters Health System (HSHS), which sponsors 13 hospitals in 12 communities across Illinois and Wisconsin, is migrating to an integrated information system and enterprise solution for the sites. Epiphany's cardio server was chosen as the enterprise-wide electrocardiogram (ECG) management system for HSHS.
An estimated 5,000 nuclear medicine and molecular imaging professionals are expected to gather in June for the 2013 Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI) to discuss the latest advances in radiotracers, imaging modalities, radionuclide therapies and quality.
InTouch Health has announced the launch of the ControlStation (CS) App for iPad, making it easier for doctors to provide real-time, acute telemedicine consults with patients. From a single interface, doctors can now connect from iPads and iPad Minis to provide patient care through any of the Company’s Food and Drug Administration (FDA)-cleared, purpose-built remote presence devices. An interface designed specifically for acute care telemedicine, it integrates clinical patient data and medical radiology imaging tools through a single user login.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Olympus received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Articulating HD 3-D Laparoscopic Surgical Video System. It delivers value to surgeons and patients by reducing surgical errors and improving the speed, accuracy and precision of surgical tasks such as dissection, grasping and suturing when compared with traditional 2-D surgical systems, based on internal Olympus testing conducted using a simulated surgical model. This is accomplished by restoring natural 3-D vision and depth perception when performing laparoscopic procedures and is independent of a surgeon’s skill level.
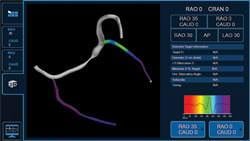
Medtronic Inc. announced market release of the CardioGuide Implant System, a novel real-time navigation system for cardiac resynchronization therapy pacemakers and defibrillators (CRT-P and CRT-D), in the United States and Canada. The system helps physicians determine the most appropriate location for left-ventricular lead placement by generating 3-D images of the cardiac veins; enhanced software for the system will be commercially available later this year that also analyzes the motion of select cardiac vessels on the left side of the heart. Clinical studies have shown that appropriate left-ventricular lead placement may improve CRT response in heart failure patients.
CardioFocus Inc. announced that as of late 2012, more than 1,000 clinical cases have been performed worldwide using the HeartLight technology. The company hosted an expert symposium to review the clinical application of HeartLight and key observations made during the first 1,000 cases in January.


 April 12, 2013
April 12, 2013