April 10, 2013 — Medtronic Inc. announced market release of the CardioGuide Implant System, a novel real-time navigation system for cardiac resynchronization therapy pacemakers and defibrillators (CRT-P and CRT-D), in the United States and Canada. The system helps physicians determine the most appropriate location for left-ventricular lead placement by generating 3-D images of the cardiac veins; enhanced software for the system will be commercially available later this year that also analyzes the motion of select cardiac vessels on the left side of the heart. Clinical studies have shown that appropriate left-ventricular lead placement may improve CRT response in heart failure patients.
Paieon Inc. is the developer of the CardioGuide system. Based on its expertise in the field of real-time cardiac fluoroscopy-based image navigation, Paieon has invested considerable engineering efforts in the development of the CardioGuide system. Medtronic has an exclusive licensing agreement with Paieon to market the CardioGuide System worldwide.
“The future of LV lead implantation requires an individual-patient-tailored approach. We know from studies that the best location for one patient can be the least effective for another,” said Raymond Yee, M.D., director, arrhythmia service, London Health Sciences Centre, Ontario, Canada. “This system provides clinical and physiologic data acquired during the CRT case to ensure that the optimal lead and tools are used to implant the lead at the targeted location, potentially helping improve patient response to the therapy.”
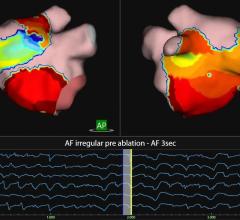
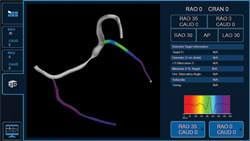
CRT is a safe and effective treatment for indicated heart failure patients, proven to significantly reduce mortality and heart failure hospitalization rates. However, approximately one-third of patients who receive a CRT device do not fully benefit from this therapy; appropriate lead placement may improve patient outcomes. The CardioGuide System’s 3-D imaging software projects a roadmap and the targeted lead placement onto an overlay of the patient’s heart, and tracks the real-time location of the electrodes within the body using fluoroscopy. This enables the implanting physician to confirm that the lead is placed in the targeted location.
In addition, left-ventricular lead positioning is known to be time-consuming for physicians. In approximately 43 percent of cases, patient procedure times extend to two hours or more. Given its ability to guide lead placement in real-time, the CardioGuide System may help reduce overall implant procedure times.
“The CardioGuide Implant System offers the medical community a viable, cost-effective solution for targeting left-ventricular lead placement, the cornerstone of successful CRT therapy,” said David Steinhaus, M.D., vice president and general manager, heart failure, and medical director for the cardiac rhythm disease management business. “This is yet another example of our commitment to offering physicians the most advanced medical technology to help improve CRT response and ensure the best quality of care for all heart failure patients.”
The CardioGuide System is part of a comprehensive approach Medtronic has undertaken to deliver solutions to optimize CRT response and manage heart failure patients at every stage of care. The system is compatible with all Medtronic CRT devices, including the Viva/Brava portfolio available in Europe. The system received a Cardiostim Innovation Award from the European Society of Cardiology in 2012, and is available in the United States and Canada.
For more information: www.medtronic.com


 September 05, 2025
September 05, 2025