March 15, 2024 —Renowned cardiovascular surgeon Gilbert H. L. Tang, MD, has been named Editor-in-Chief of JACC: Case ...
March 15, 2024 — Following the recent CE approval of the Solia S lead1 for LBBAP, Biotronik announces the world’s first ...
March 15, 2024 — BioCardia, Inc., a biotechnology company focused on advancing late-stage cell therapy interventions for ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
March 15, 2024 —Renowned cardiovascular surgeon Gilbert H. L. Tang, MD, has been named Editor-in-Chief of JACC: Case ...
March 14, 2024 — The American Medical Association (AMA) has issued a new letter to federal officials in which it praised ...
March 13, 2024 — In the largest randomized clinical trial and first of its kind to date in the United States, a team led ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
March 13, 2024 — BioCardia, Inc., a developer of cellular and cell-derived therapeutics for the treatment of ...
March 13, 2024 — Two nationally recognized cardiovascular programs, New Jersey-based Atlantic Health System’s Morristown ...
March 13, 2024 — Silence Therapeutics has announced positive topline 36-week data from the ongoing ALPACAR-360 phase 2 ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
March 12, 2024 — Medtronic plc, a global leader in healthcare technology, today announced two late-breaking data ...
March 12, 2024 — Stereotaxis, a pioneer and global leader in surgical robotics for minimally invasive endovascular ...
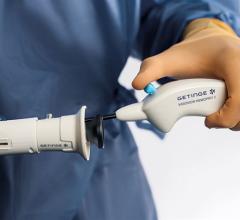
March 12, 2024 — Getinge has announced the U.S. Food and Drug Administration’s (FDA) 510(k) clearance of the Vasoview ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
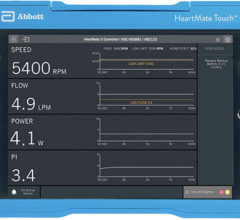
March 12, 2024 — The U.S. Food and Drug Administration (FDA) announced that Abbott is recalling its HeartMate Touch ...
March 11, 2024 — VahatiCor, Inc., a med tech company dedicated to helping patients with persistent ischemic heart ...
March 8, 2024 — The Texas Heart Institute, Georgia Institute of Technology (Georgia Tech), North Carolina State ...


 March 15, 2024
March 15, 2024