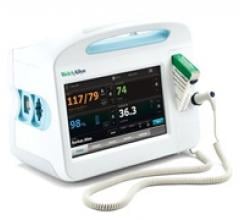
February 11, 2011 – A technology integration strategy will provide clinicians with a host of new noninvasive, continuous measurements and patient-monitoring capabilities. The joint strategy, between Welch Allyn and Masimo, will make the Masimo rainbow SET Pulse CO-Oximetry platform a fundamental offering in Welch Allyn’s product line-up.
February 11, 2011 – A power-injectable, extended-dwell catheter has gotten the CE Mark. The Powerwand, from Access Scientific, is placed using the Accelerated Seldinger Technique, and promises to improve the inpatient experience while increasing worker safety.
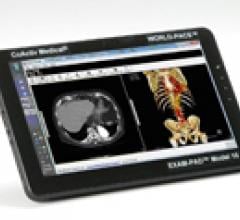
February 11, 2011 — Exam-Pad Model 10 is the first turnkey picture archiving and commuication systems (PACS) tablet that combines instant, virtual, worldwide wireless access to medical images in a Windows 7 Professional environment.
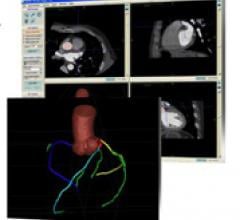
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
February 10, 2011 – A study at Millard Fillmore Circle Gates Hospital in Buffalo, N.Y., found that Toshiba imaging equipment presented significant benefits, both for the patients and hospital.
February 10, 2011 – A new bill introduced to the U.S. Senate aims to promote sustainable, domestic production of the medical isotope molybdenum-99 (Mo-99). The bill, also known as the American Medical Isotopes Production Act of 2011, was introduced by Sen. Jeff Bingaman (D-N.M.) and Sen. Lisa Murkowski (R-Ark.). Bingaman is chair of the Senate Committee on Energy and Natural Resources.
February 10, 2011 – Only about two out of every 100 babies (1.8 percent) are screened at birth for congenital heart defects (CHDs) because the hospital or birthing center does not routinely screen all newborns. CHDs are the No. 1 birth defect and leading killer of infants and newborns.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
February 10, 2011 – Never before has a therapy proven more beneficial for women than men in preventing heart disease – until now.
February 10, 2011 – A Phase 2 clinical trial has been initiated to test the safety and efficacy of drug to reverse heparin in patients undergoing percutaneous coronary intervention (PCI). The trial will assess PolyMedix’s heptagonist, PMX-60056, a synthetic small-molecule designed to reverse the anticoagulation activity of heparin and low molecular weight heparins (LMWH).
February 9, 2011 – The first patients have been enrolled in a trial investigating the safety and efficacy of a system that removes clot from acute ischemic stroke patients. The Trevo 2 study will look at the Trevo Retriever device, from Concentric Medical. It is the first device to use Stentriever technology for retrieving clots from the neurovasculature.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
February 9, 2011 – The U.S. Food and Drug Administration (FDA) granted 510(k) clearance for enhanced software that automatically detects significant (50 percent and more) stenotic lesions in coronary arteries from coronary computed tomography angiography (CTA) studies. The COR Analyzer system, from Rcadia Medical Imaging, is designed to help speed detection of coronary artery disease.
February 9, 2011 – The U.S. District Court for the District of Delaware reaffirmed an April 2010 federal jury decision that determined Medtronic CoreValve LLC willfully infringed Edwards Lifesciences' U.S. Andersen transcatheter heart valve patent and awarded Edwards $74 million in damages.
February 9, 2011 – The U.S. Food and Drug Administration (FDA) said certain lots of the Arstasis One femoral artery access system are part of a Class 1 recall. Components of the device may fracture and/or separate during use, which may result in patient harm, the FDA said.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
February 9, 2011 – A partnership has been formed to launch a portable therapeutic cooling system in Europe. Physio-Control and BeneChill will work together to launch the RhinoChill IntraNasal Cooling System, a noninvasive system for transnasally lowering the body’s core temperature immediately following cardiac arrest, stroke or traumatic brain injury
February 9, 2011 – The U.S. Food and Drug Administration (FDA) approved the first pacemaker in the United States specifically designed for use in an magnetic resonance imaging (MRI) environment and approved as MRI-conditional. Medtronic said its Revo MRI SureScan pacing system is available immediately.

Interoperability is key to the meaningful use and sharing of patients’ electronic images and information.

 February 11, 2011
February 11, 2011