February 11, 2011 – A technology integration strategy will provide clinicians with a host of new noninvasive, continuous measurements and patient-monitoring capabilities. The joint strategy, between Welch Allyn and Masimo, will make the Masimo rainbow SET Pulse CO-Oximetry platform a fundamental offering in Welch Allyn’s product line-up.
In addition, the upgradeability of the rainbow SET technology platform, will allow clinicians the option to remotely add Masimo's noninvasive and continuous measurements as optional software upgrades.
"Welch Allyn is excited to provide our customers with the option of enabling the Masimo parameters within our patient monitoring devices in a manner that considers their evolving needs over time," said Welch Allyn Executive Vice President and Chief Global Business Officer, Steve Meyer. "Masimo technology supports our platform concept of modularity and upgradeability while allowing customers to improve patient care through early, noninvasive continuous and spot check monitoring of key hemodynamic parameters."
Masimo rainbow SET noninvasive and continuous measurements provide detailed physiological data that helps clinicians to more rapidly assess patients and detect adverse conditions earlier.
Immediate availability of detailed clinical intelligence and measurement data lets clinicians proactively detect and treat the early signs of hemodynamic instability, potentially life-threatening conditions and internal bleeding.
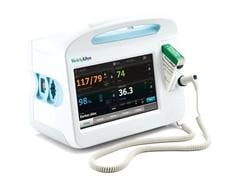
The new Welch Allyn Connex Vital Signs Monitor (CVSM) is the company’s first monitor to integrate the Masimo rainbow SET technology. The CVSM, which launched in August 2010, incorporates Masimo's SpO2-enabled measurements and is capable of being field upgraded.
The CVSM is a full-color, touch screen device that acts as three devices in one, providing comprehensive patient documentation on a single display. This documentation includes automatic measurements such as heart rate, blood pressure, temperature and pulse oximetry. It also includes manual parameters such as respiration, height, weight and pain level, and modifiers such as body position, O2 therapy details and others. The device also gives the clinician the ability to control alarms, patient data and monitoring in a customized manner for each patient, and document the data right on the device. This eliminates the need to locate a PC and transcribe it later.
For more information: www.welchallyn.com, www.masimo.com


 October 21, 2025
October 21, 2025 









