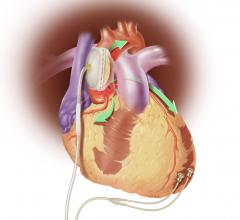
Nanometer-sized “drones” could become a new way to prevent heart attacks caused by atherosclerosis, according to a study in pre-clinical models by scientists at Brigham and Women’s Hospital (BWH) and Columbia University Medical Center. The drones could be used to deliver a special type of healing molecule to fat deposits in arteries, according to findings published in the February 18th online issue of Science Translational Medicine.
Patients who experienced a certain type of heart attack who received the anticoagulant fondaparinux had a lower risk of major bleeding events and death compared to patients who received low-molecular-weight heparin (LMWH), according to a study in the February 17 issue of the Journal of the American Medical Association (JAMA). This was found to be true both in the hospital and after six months; however, both groups had similar rates of subsequent heart attack or stroke.
By loading magnetic nanoparticles with drugs and dressing them in biochemical camouflage, Houston Methodist researchers say they can destroy blood clots 100 to 1,000 times faster than a commonly used clot-busting technique. The finding, reported in Advanced Functional Materials, is based on experiments in human blood and mouse clotting models.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
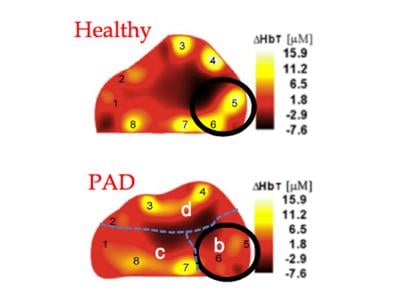
Approximately 8 to 12 million people in the United States alone are suffering from peripheral arterial disease (PAD), a common vascular problem that is also one of the most serious complications of diabetes. Andreas Hielscher, professor of biomedical engineering, electrical engineering and radiology (physics) at Columbia Engineering, is developing a novel technology that could improve diagnosis of this crippling disease and make it easier to monitor patients.
The ScottCare Corp. announced its new novi patch Holter monitor will be available in the United States for the first time at the 2015 Scientific Sessions of the American College of Cardiology in San Diego, Calif., March 14-16.
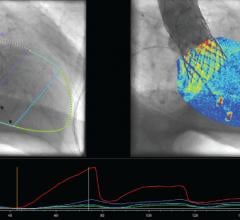
CBSET, a not-for-profit preclinical research institute, announced that its scientists have defined the optimal tissue debulking protocol for treating in-stent restenosis (ISR) with Boston Scientific’s Jetstream Navitus atherectomy device. These findings were disclosed at the Cardiovascular Research Technologies 2015 annual scientific meeting (CRT 2015) in Washington, D.C.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Sunshine Heart Inc. announced it has received unconditional approval from the U.S. Food and Drug Administration (FDA) to conduct an interim analysis of COUNTER HF, the company's U.S. pivotal study.
SuperSonic Imagine announced a partnership with U.S.-based Unetixs Vascular Inc. to bring SuperSonic Imagine’s technology to more customers in the vascular market.
In light of a recent American Heart Association report stating that U.S. mitral valve regurgitation prevalence was 1.7 percent in 2014, a significant opportunity exists for development of an effective transcatheter mitral valve replacement (TMVR) device as an alternative treatment to surgery for inoperable patients. This assessment was given by an analyst with research and consulting firm GlobalData.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Daiichi Sankyo and the Heart Rhythm Society announced results from a global survey, which polled cardiologists from around the world and revealed that a majority (58 percent) agree there is no such thing as a "typical" non-valvular atrial fibrillation (NVAF) patient.
The National Forum for Heart Disease & Stroke Prevention announced the launch of the Stronger Hearts Helpline, a 24/7 free call-center resource for people with heart failure and their families. The pilot program is an addition to the existing 2-1-1 information and referral hotline in San Bernardino County, California – a region where nearly 20 percent of Medicare recipients are being treated for heart failure – and long-term plans are to expand the service nationwide.
Americans are ready and willing to leverage health apps and wearable devices to improve their personal health, according to the findings released from the fifth annual Makovsky/Kelton "Pulse of Online Health" Survey.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Medtronic announced that the U.S. Centers for Medicare and Medicaid Services (CMS) has approved a transitional pass-through payment for the company’s IN.PACT Admiral drug-coated balloon (DCB) under the Medicare hospital outpatient prospective payment system (OPPS). The decision removes a potential barrier to patient access to this new medical device, which represents an improvement to the standard of care for peripheral arterial disease in the upper leg.
C. R. Bard Inc. announced that the U.S. Centers for Medicare and Medicaid Services (CMS) has approved a pass-through payment for the Lutonix drug-coated balloon (DCB) under the Medicare hospital outpatient prospective payment system. This approval follows a unanimous favorable recommendation from the U.S. Food and Drug Administration’s (FDA) Circulatory Systems Devices Advisory Panel.
Pie Medical Imaging BV announced that it received 510(k) clearance from the U.S. Food and Drug Administration for its CAAS A-Valve product including the quantitative Regurgitation Analysis (qRA) workflow. The qRA workflow is the first 510(k) cleared image analysis technology to determine aortic regurgitation based on X-ray angiography.

 February 26, 2015
February 26, 2015