April 22, 2011 – The first patient has been treated in a European multicenter CIRCUS trial. The study is looking at NeuroVive Pharmaceutical’s CicloMulsion cremophor-free IV cyclosporine formulation in 1,000 patients undergoing percutaneous coronary intervention (PCI) for acute myocardial infarction.
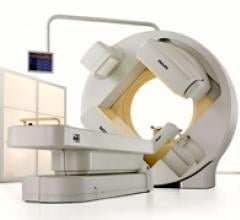
April 22, 2011 – At the Society of Nuclear Medicine’s annual meeting, Philips will highlight several new imaging systems. The meeting will take place June 4-8 in San Antonio, Texas.
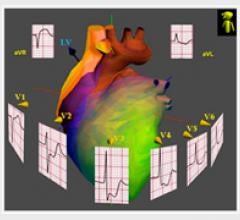
April 22, 2011 – The American Diabetes Association (ADA) has invited NewCardio to present the results of a key study of the company’s urgent care solution, my3KG. Results showed that the solution had substantially greater accuracy than expert cardiologists' interpretation of standard 12-lead ECG (12L ECG) in diagnosing acute myocardial infarction (AMI) in diabetic patients.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
April 22, 2011 – A new study found that coronary artery bypass surgery added to medical therapy for selected chronic heart failure patients offered benefits over medical therapy alone.
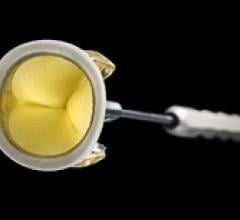
April 22, 2011 – The U.S. Food and Drug Administration (FDA) has cleared the Trifecta valve, by St. Jude Medical. The valve, a clinically-proven replacement for diseased, damaged or malfunctioning aortic heart valves, mimics the flow of a natural, healthy heart.
April 22, 2011 – The Society of Interventional Radiology (SIR) has a long-term commitment to radiation safety, taking a leading role in measuring and assessing radiation dosage; developing educational programs on radiation safety, radiation protection and reduction of skin dosage; and promoting the safety of patients and healthcare professionals.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
April 21, 2011 – A new intravascular ultrasound (IVUS) coronary imaging system has received the CE Mark. With the approval, the LipiScan IVUS system, from InfraReDx, is available for the detection of the plaques known to complicate stenting and believed to be the reason for most heart attacks.
April 21, 2011 – ScImage announced the launch of a project to assist the United States Air Force (USAF), Aeromedical Consultation Service (ACS) with the collection, assessment and reporting tools necessary to expedite cardiovascular decisions for rated aviators, while concurrently reducing flight disqualification delays.
April 21, 2011 – A new angiotensin II receptor blocker (ARB) has been approved by the U.S. Food and Drug Administration (FDA) to treat hypertension, or high blood pressure. Edarbi (azilsartan medoxomil), by Takeda Pharmaceutical Company, is now available by prescription for adults in U.S. pharmacies.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
April 21, 2011 – Baxter International has entered into a definitive agreement to acquire privately-held Prism Pharmaceuticals, a specialty pharmaceutical company based in King of Prussia, Penn. Prism Pharmaceuticals has developed and received U.S. Food and Drug Administration (FDA) approval for multiple presentations of Nexterone (amiodarone HCl), an antiarrhythmic agent.
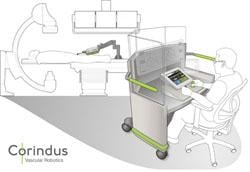
The start-up company Corindus for several years has been developing a robotic-assist system for use in the cardiac cath lab. It not only navigates devices to target lesions, but also can be used to precisely guide, manipulate and deploy devices during interventional procedures.
April 21, 2011 - Philips Healthcare and Corindus Inc. today announced an alliance agreement to add Corindus’ robotic-assisted cath lab navigation system for percutaneous coronary interventions (PCI) to Philips’ interventional cardiology solutions.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
April 20, 2011 – Patients who have aneurysms, stroke, heart rhythm disorders, cholesterol build up in arteries and numerous other cardiovascular and neurovascular diseases will benefit from new technology now in place at Methodist University Hospital. The technology produces 3-D images that are faster and more accurate, and that aid advanced surgery and treatments.
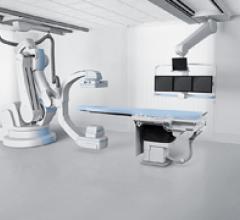
April 20, 2011 – Philips has announced the world’s first hybrid operating room (OR) with the new FlexMove - a ceiling-mounted option for the Allura Xper X-ray system at the Hospital Zürich (UniversitätsSpital Zürich, USZ), Switzerland.
A new PET/CT scanner has been introduced that helps address the increasing cost pressures faced by healthcare providers. GE Healthcare’s Optima PET/CT 560 combines superb image quality, high productivity and a patient-friendly experience that can continuously deliver ongoing value over time.


 April 22, 2011
April 22, 2011