In the same fashion that the echocardiogram helped transform the cardiac exam, recent developments in the field of portable ultrasound are providing cardiologists with a new way to visualize the heart at the initial patient screening.

In a world where everything is unplugged and cordless is king, cardiology is constantly searching for and developing the technology to make patient care both better and more convenient.

For patients with severe hypertension that remains uncontrolled despite medical therapy, radiofrequency (RF) energy can be used in the renal vessels to block nerves involved with the sympathetic nervous system. Researchers believe this has the potential to impact the mechanical and hormonal activities of the kidneys and may eliminate the need for medical therapy.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Over the past few years, there have been three key areas of advancement in cardiovascular ultrasound technology – miniaturization, 3-D/4-D imaging and quantification measurements.
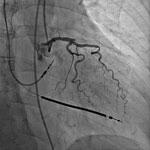
When it comes to interventional cardiology, increasing diagnostic confidence and reducing exam time are paramount to improving overall patient care.
December 10, 2010 – A new set of software applications that improves the quality of patient data and increases clinician productivity and efficiency has been launched. SentrySuite, by CareFusion, is designed for pulmonary and cardiopulmonary diagnostic settings. The software focuses on data, organization, workflow and connectivity so physicians can focus more time on patient care.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
December 10, 2010 – An ECG management system is now offered with an IT solution for cardiac imaging. Siemens Healthcare now offers the ScImage PicomECG with its syngo Dynamics system.
December 10, 2010 – The 5th annual list of the Top 10 Health Technology Hazards for 2011 is now available. The list, from the ECRI Institute, features the top health technology hazards that warrant critical attention by hospitals and other healthcare organizations.
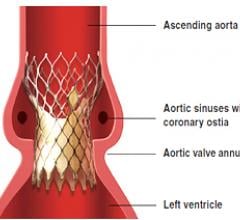
December 10, 2010 – European CE mark approval has been granted so the Medtronic CoreValve transcatheter aortic valve replacement system can be delivered through the subclavian artery, located beneath the collar bone.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
December 10, 2010 – The first commercial production of generators using molybdenum-99 (Mo-99) produced with low-enriched uranium (LEU) targets in the United States is underway. The Lantheus TechneLite (Technetium Tc99m) generator received the first commercial scale batch from NTP Radioisotopes, a subsidiary of the Nuclear Energy Corporate of South Africa (NESCA)
December 9, 2010 – A cardiovascular image and data management system (CIIMS) has been successfully implemented at nine Iowa Health System (IHS) affiliate hospitals. HIS used the enterprise-wide Lumedx CardioInventory system.
Tony Pinson, RTRCV, has worked at Carroll Hospital Center in Westminster, Md., for nearly 20 years. During that time, the cardiac catheterization laboratory supervisor has witnessed drastic changes at the private, nonprofit, 213-bed hospital that prides itself on providing exceptional care for patients in Carroll and surrounding counties.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Over the past several years, the focus on new technology at the annual Radiological Society of North America (RSNA) scientific meeting in Chicago has moved away from new devices to new software. RSNA 2010, held Nov. 28 through Dec. 2, emphasized this trend.
December 7, 2010 - In an effort to improve the diagnosis and treatment of peripheral artery disease (PAD), the American College of Cardiology and American Heart Association issued the first-ever performance measures for adults with PAD. These new performance measures also aim to increase understanding of the serious heart-related effects of PAD within the healthcare community.
December 7, 2010 – A study comparing a transcatheter ventricular assist device (VAD) to intra-aortic balloon pumps (IABP) has been completed after interim analysis determined the primary endpoint was likely. The Impella, by Abiomed, significantly reduced major adverse events out of hospital.

 December 13, 2010
December 13, 2010