Jan. 27, 2026 — Robocath has launched the world’s first FIH (First-In-Human) clinical study evaluating its new robotic ...
Jan. 26, 2026 — AISAP, a provider of AI point-of-care diagnostics, has published a new clinical study in the peer ...
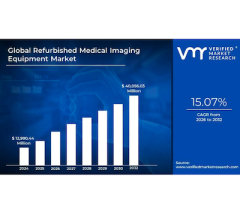
Jan. 11, 2026 — The Global Refurbished Medical Imaging Equipment Market Size is projected to grow at a CAGR of 15.07% ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Jan. 20, 2026 — Abbott has received CE Mark in Europe for the TactiFlex Duo Ablation Catheter, Sensor Enabled to treat ...
Jan. 20, 2026 — Kardium Inc. has announced the publication of the PULSAR clinical trial results in the Journal of the ...
Jan. 20, 2026 — Polarean, a commercial-stage medical imaging company advancing functional MRI of the lungs, has expanded ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
On Feb. 19, at 7 p.m. eastern time, Cleerly will present a live webinar on “Women's Heart Health: Uncovering Hidden ...
Jan. 15, 2026 — Boston Scientific Corp. and Penumbra, Inc. have entered into a definitive agreement under which Boston ...
Jan. 16, 2026 — MemorialCare Heart & Vascular Institute has launched the FDA-approved Symplicity Renal Denervation (RDN) ...
For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet ...
Jan. 6, 2026 — UltraSight, a provider of AI-guided cardiac imaging workflows, has announced FDA clearance to expand its ...
Jan. 13, 2026 – Innovative Health, Inc. has received its 50th clearance from FDA to reprocess single-use medical devices ...
Jan. 13, 2026 — A streamlined, nurse-led response for hospitalized patients experiencing an acute stroke at a Texas ...
In the United States, the options currently available for cardiac ablation use thermal mechanisms to ablate tissue and ...
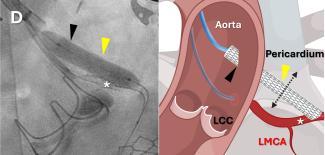
In a world first, a team of researchers at the National Institutes of Health (NIH) and Emory School of Medicine, Atlanta ...
Jan. 8, 2026 — AccurKardia recently announced U.S. Food and Drug Administration (FDA) 510(k) clearance and the ...
Jan. 13, 2026 — AliveCor has received U.S. Food and Drug Administration (FDA) clearance for the next generation of KAI ...


 January 27, 2026
January 27, 2026