The Vivid e adds a fully integrated, three-connector, docking cart, creating a true hybrid system with the power of ...
The LIFEPAK 1000 defibrillator/monitor is the most rugged defibrillator ever designed by Physio-Control, with an IP55 rating. ADAPTIV biphasic technology provides a range of energy up to 360 joules, and an upgradeable platform. The 1000 is equipped with cprMAX technology, enabling care providers to change protocols as standards of care evolve.
March 18, 2008 - Northeast Monitoring Inc. released LX Sleep, a software product that reportedly detects OSA from data collected during a simplified, overnight, at-home, patient hookup to its OxyHolter product, as the company plans to launch its product line worldwide.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
McKesson has released a new Horizon Cardiology product for the cath lab, the Holding Area Charting, designed to simplify and expedite information flow in the cath lab, increase data accuracy by minimizing duplication and strengthen the facility’s commitment to patient safety.
March 18, 2008 – Today Bristol-Myers Squibb Medical Imaging introduced its new name by launching Lantheus Medical ...
Northeast Monitoring has introduced the DR200/HE, a combination 14-day Holter plus 30-day Event recorder integrated into ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
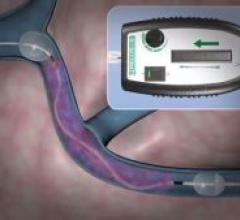
March 18, 2008 - A catheter-based device, the Trellis-8 infusion catheter, which disperses thrombolytic solution ...
March 18, 2008 - Northeast Monitoring has introduced the DR200/HE, a combination 14-day Holter plus 30-day Event ...
Bacchus Vascular Inc. has developed a catheter-based device, the Trellis-8 infusion catheter, which disperses ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
NorthEast Monitoring Inc. released LX Sleep, a software product that reportedly detects OSA from data collected during a simplified, overnight, at-home, patient hookup to its OxyHolter product.
March 18, 2008 – The FDA gave AtriCure’s Coolrail linear ablation pen 510(k) clearance for the ablation of cardiac ...
March 18, 2008 – Northeast Monitoring received clearance from the FDA to sell its DR200 Series devices with its ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
AtriCure’s Coolrail linear ablation pen for the ablation of cardiac tissue can be used for patients with persistent and ...
March 18, 2008 - McKesson reports that it is unveiling two new Horizon Cardiology products for the cath lab, including ...
March 11, 2008 - Mindray Medical International Ltd. signed a definitive agreement to acquire Datascope Corp.’s patient ...

 March 19, 2008
March 19, 2008